Solid dosage forms, e.g., tablets, capsules etc., are by far the most implemented drug formulation types Worldwide. Therefore, in pharmaceutical research, unraveling the intricate interplay between dissolution and absorption is paramount for refining drug delivery strategies and maximizing therapeutic outcomes.
Dissolution, the process whereby solid compounds transition into solution form, stands as a pivotal determinant in the absorption kinetics of orally administered drugs. This fundamental understanding serves as a cornerstone for advancing drug development not only in human medicine but also in nutraceutical and veterinary sectors.
Leveraging innovative technologies such as MIVO®, as demonstrated in the study by Ass. Professor Di Cagno and his group at University of Oslo, presents a promising avenue for advancing dissolution and permeation studies1. This integration offers simultaneous data acquisition, enhanced predictability, cost reduction, and streamlined experimental procedures, revolutionizing drug development processes. In other words, this might be a promising gateway for the green and sustainable transition pharmaceutics is facing.
Dissolution’s Influence on Absorption:
The gastrointestinal tract serves as the primary site for drug absorption following oral administration. However, before drugs can exert their pharmacological effects, they must undergo dissolution to facilitate absorption into systemic circulation.
The rate and extent of dissolution profoundly impact the bioavailability, onset of action, and efficacy of drugs. Hence, optimizing dissolution characteristics is paramount for ensuring the therapeutic effectiveness of orally administered medications.
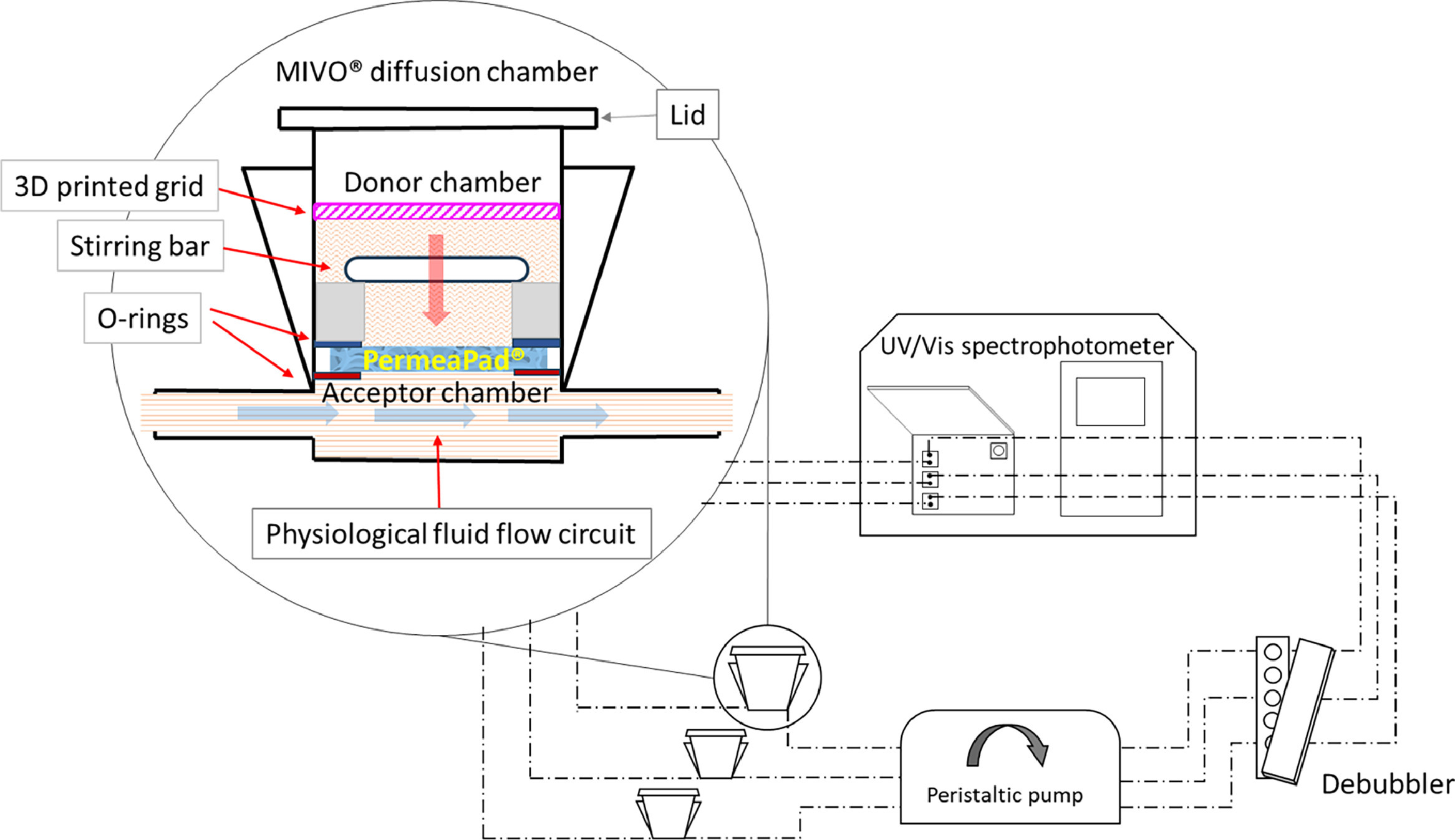
Traditionally, dissolution and permeation studies have been conducted separately, resulting in time-consuming and resource-intensive processes. However, MIVO® technology, in the MCPA (Meso-fluidic Chip for Permeability Assessment) configuration enables concurrent measurement of dissolution and permeation parameters, offering real-time insights into drug behavior. This simultaneous assessment, together with use of Permeapad® biomimetic membrane, enhances experimental predictability and efficiency, enabling researchers to make informed decisions regarding drug candidates and formulations.
Strategies for Enhancing Dissolution and Absorption:
Over the years, researchers have devised innovative formulation strategies to enhance dissolution kinetics and subsequent absorption. Co-crystallization techniques, for instance, offer a means to modulate the physicochemical properties of active pharmaceutical ingredients (APIs), thereby improving their solubility and dissolution rates without compromising therapeutic efficacy. Similarly, amorphous solid dispersions (ASDs) have emerged as promising formulations for stabilizing supersaturated solutions, consequently enhancing drug bioavailability. These advancements underscore the importance of leveraging formulation science to overcome solubility challenges and optimize oral drug delivery.
One of the key advantages of this MIVO®-based technology is its ability to operate with small sample volumes, typically in the milliliter range. The reduced sample volume not only minimizes resource consumption but also translates to cost savings, making this novel system an economically viable option for drug development. Furthermore, the compact design eliminates the need for extensive laboratory space, optimizing research facility utilization. Also, by providing comprehensive dissolution and permeation data in real time, the technology enables researchers to refine drug formulations during the design phase. This proactive approach enhances formulation quality, leading to improved product performance and efficacy. Moreover, the predictive nature of MIVO® technology allows for early identification of promising formulations, streamlining subsequent optimization efforts.
Applications in Drug Development and Beyond:
The implications of dissolution extend beyond traditional pharmaceuticals to encompass a broader spectrum of products, including nutraceuticals and veterinary treatments. In the realm of nutraceuticals, optimizing dissolution properties is crucial for ensuring the bioavailability of essential nutrients, vitamins, and minerals. Similarly, in veterinary medicine, enhancing dissolution can significantly improve the efficacy of orally administered therapies and products in animals. By harnessing principles of dissolution science across diverse sectors, researchers can innovate novel formulations with enhanced absorption and therapeutic efficacy.
Breaking New Ground in Solid Dosage Formulation Testing Integrated MIVO® Technology:
The integration of MIVO® technology in the MCPA system, revolutionizes dissolution and permeation studies, offering a comprehensive and efficient approach to solid dosage form formulation testing. Through simultaneous data acquisition, cost-effective operation, and streamlined procedures, it accelerates screening processes, enhances formulation design, and ultimately contributes to the advancement of therapeutic interventions.
In conclusion, the relationship between dissolution and absorption stands as a cornerstone in drug development, transcending boundaries across pharmaceutical, nutraceutical, and veterinary industries. Through meticulous optimization of dissolution properties via innovative formulation approaches, researchers can unlock the full potential of oral drug delivery, thereby improving patient outcomes and advancing healthcare paradigms.
Reference:
1Tzanova MM, Larsen BS, Birolo R, Cignolini S, Tho I, Chierotti MR, Perissutti B, Scaglione S, Stein PC, Hiorth M, Di Cagno MP. Shifting the Focus from Dissolution to Permeation: Introducing the Meso-fluidic Chip for Permeability Assessment (MCPA). J Pharm Sci. 2023 Dec 16:S0022-3549(23)00538-5.

