MIVO® organ-on-chip
More Reliable, Robustand Predictive Outcomes.

Overcoming the limitations of traditional cell culture methods
Current methodologies, both in vitro and animal ones, are limited in their ability to mimic the extremely complex processes of human physiology.
As a result, both of them frequently fall short of being translated in human disease understanding and effective therapies development.
A new and more reliable tool is now available to help researchers in studying the complexity of human biology, both in physiological and pathological conditions
With MIVO® organ-on-a-chip, every static tissue model may be converted to dynamic. Set up your fully controlled millifluidic environment working on relevant tissue size to deepen the mechanisms behind tissue regeneration and disease progression, and get more predictive insights.
Do you want to take it to the next level? Discover TOKYO – the unique high performing millifluidic platform.
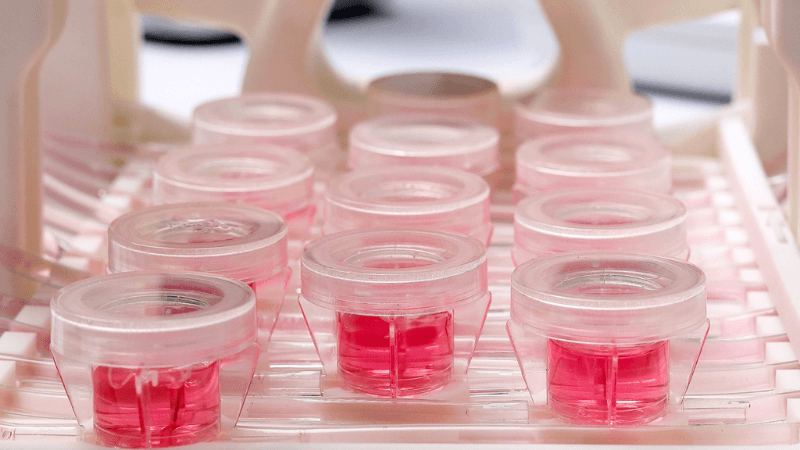
2D & 3D DYNAMIC TISSUE CULTURE
MIVO® TECHNOLOGY
Physiological flow
Each human district has its own flow rate and fluid flow-induced shear stress strongly influences cell behavior. A complete control of these parameters is essential to achieve more predictive results and to recapitulate physiological conditions.

3D tissues/patient biopsies
3D cultures are more physiologically relevant and predictive compared to the oversimplified 2D standard culture, giving a more realistic sight of cell-cell and cell-matrix interactions and drugs & molecules diffusion.

Multi-organ connection
The fluidic connection among multiple tissues enables to recapitulate in vitro the cross-talk among different organs, being crucial for systemic diseases models, pharmacokinetic and pharmacodynamic studies

Easy cells/media sampling
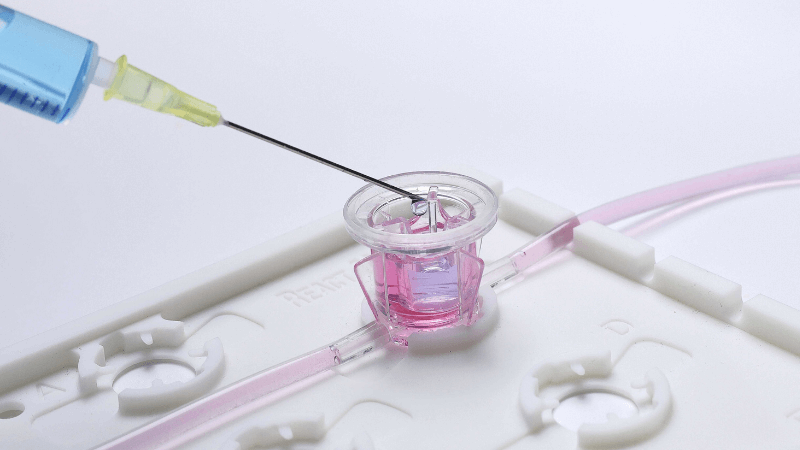
A three-way valve and an open lid allow to easily inject/sample cells and media from lower and upper tissue cultures compartments at different time points, for multiple readouts.

Customizable & Flexible
Keep your established cellular models, either in 2D monolayer or in 3D, and easily convert your protocol assays into the MIVO® device among the wide range of fluidic configurations.

Quick & Easy
Disposable and sterile tissue chambers can be assembled in less than 2 minutes without any technological skills or additional equipment investments, allowing to perform controlled and reproducible assays in vitro
MIVO® Single-Organ Platform
Single-organ models allow controlled culture of cells and tissues under physiological conditions for a deep understanding of human diseases behaviour and single organ function modelling.
MIVO® single-organ platform allows to handle separate environments (both apical and basal chambers), and different culture media and flow conditions can be fully set in terms of flow rate, PH, metabolic concentrations, and others; finally mimicking the complexity of the human tissue.
MIVO® Multi-Organ Platform
Multi-organ models are extremely challenging for studying organ-organ crosstalk affecting systemic diseases and testing molecules biodistribution. MIVO®is the unique organ on chip platform that can connect more than 3 independent chambers into realistic controlled millifluidic physiological conditions.
A cell culture model including the connection of multiple and distant organs is more predictive of in vivo conditions. It allows accurate and reproducible pharmacokinetics and pharmacodynamics assays by testing drug bio-availability and toxo-efficacy on the targeted organ and other organs.
Tissue Models Compatibility

Funcionalized Membranes
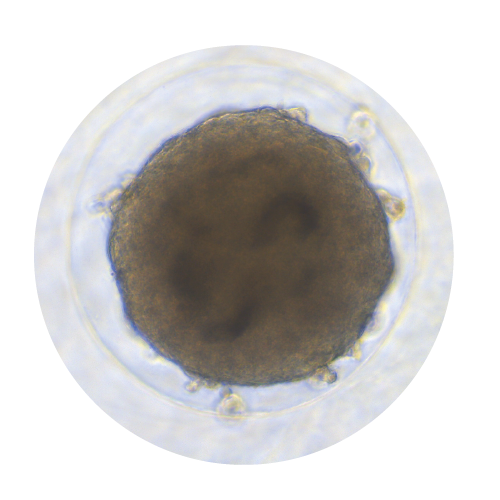
Spheroids, Organoids, and Biopsies
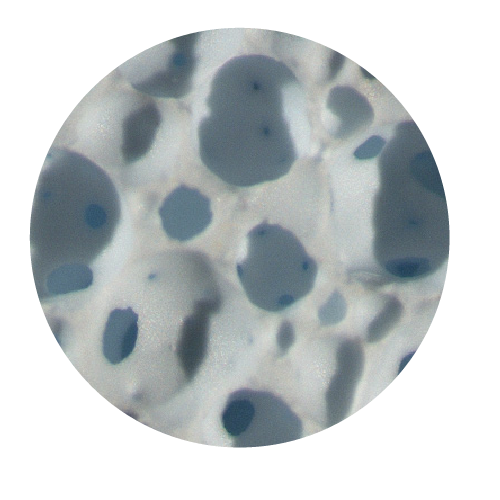
Matrixes of different dimensions & shapes
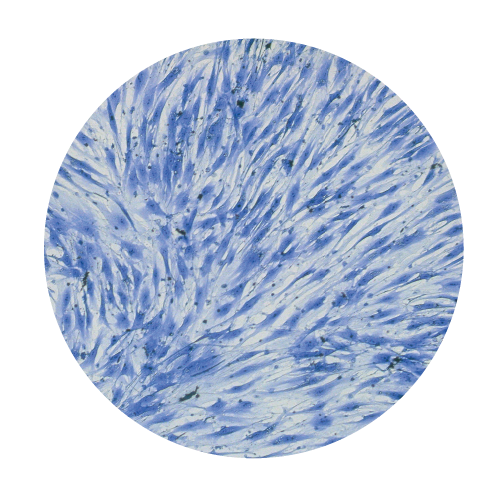
Cells Monolayers
Reconstructed Tissues

3D Hydrogels
Get a Quote
We are here for you
Whether you’re looking for a quote or want to connect with us, fill out the form below with your details, and we will be in touch shortly.
All fields are required, take your time.