The success of immunotherapeutic approaches strictly depends on the immune cells interaction with cancer cells.
While conventional in vitro cells cultures under-represent the complexity and dynamic crosstalk of the tumor microenvironment, animal models do not allow deciphering the anti-tumor activity of the human immune system.
Therefore, the development of reliable and predictive preclinical models has become crucial for the screening of immune-therapeutic approaches. We here present a novel organ-on-chip (OOC)-based approach for recapitulating the immune cell (NK) migration under physiological fluid flow, infiltration within a 3D tumor matrix, and activation against neuroblastoma cancer cells in a fully-humanized, fluid-dynamic, and clinically relevant-sized environment.
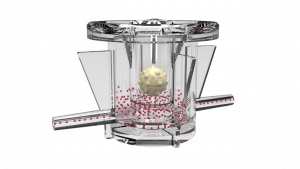
Circulating NK cells actively initiate a spontaneous and specific “extravasation” process toward the physically separated tumor niche, retaining their ability to interact with matrix-embedded tumor cells, and to display a cytotoxic effect (tumor cell apoptosis). Since NK cells infiltration and phenotype is correlated with prognosis and response to immunotherapy, their phenotype is also investigated: most importantly, a clear decrease in CD16-positive NK cells within the migrated and infiltrated population is observed.
In conclusion, this immune-tumor OOC-based model represents a challenging approach for faithfully recapitulating the human pathology and efficiently employing the immunotherapies testing, eventually in a personalized perspective
Monica Marzagalli, Giorgia Pelizzoni, Arianna Fedi3, Chiara Vitale, Fabrizio Fontana, Silvia Bruno, Alessandro Poggi, Alessandra Dondero, Maurizio Aiello, Roberta Castriconi, Cristina Bottino, and Silvia Scaglione
A multi-organ-on-chip to recapitulate the infiltration and the cytotoxic activity of circulating NK cells in 3D matrix-based tumor model – Front. Bioeng. Biotechnol.
Sec. Tissue Engineering and Regenerative Medicine
All Resources
Never stop learning!
Check publications from the team, protocols, and useful information to boost your research and get into organ on chip technology!

