Skin absorption assays are employed to assess the absorption kinetics of different molecules. Absorption critically determines the bioavailability of the compound: indeed, in order for a compound to reach a tissue, it usually needs to reach the bloodstream before exerting its biological effects. Depending on the application field, the aim of dermal absorption assays is to demonstrate the successful delivery of active ingredients, to predict the risks related to the exposure to potentially toxic molecules or to proof the absence of absorption of specific substances classes. Furthermore, dermal absorption assays could be crucial for the evaluation, classification, risk assessment and intended use of a medical device.
According to the definition of “medical device”, the latter is endowed of two main features: (i) the therapeutic and/or diagnostic medical purpose, and (ii) the main mechanism of action: non-pharmacological, neither immunological, nor metabolic. And, according to the EU Regulation 2017/745, a new classification refers to the medical devices composed of “substances or combinations of substances that are intended to be introduced into the human body through a body orifice or applied to the skin and that are absorbed by or locally dispersed into the human body”. Specifically, if this kind of devices are applied on the skin or in the nasal or oral cavity as far as the pharynx, they shall be classified as class IIa; whereas, they shall be classified as class III (highest risk) if they are systemically absorbed by the human body in order to achieve their intended purpose, or if they achieve their intended purpose in the stomach or lower gastrointestinal tract and they are systemically absorbed by the human body.
Currently, skin absorption assays are conventionally performed following the OECD428 guidelines: to align the inter-laboratory data, such guideline defines the standardization of methodologies, for reducing variability while increasing reproducibility, through a 3Rs (reduce, replace, refine) approach.
Reliable models for reducing/replacing animal tests should be able to recapitulate a biological microenvironment as physiological as possible: in this context, Franz Cells are currently used as standard diffusive chamber to perform skin permeation assays.
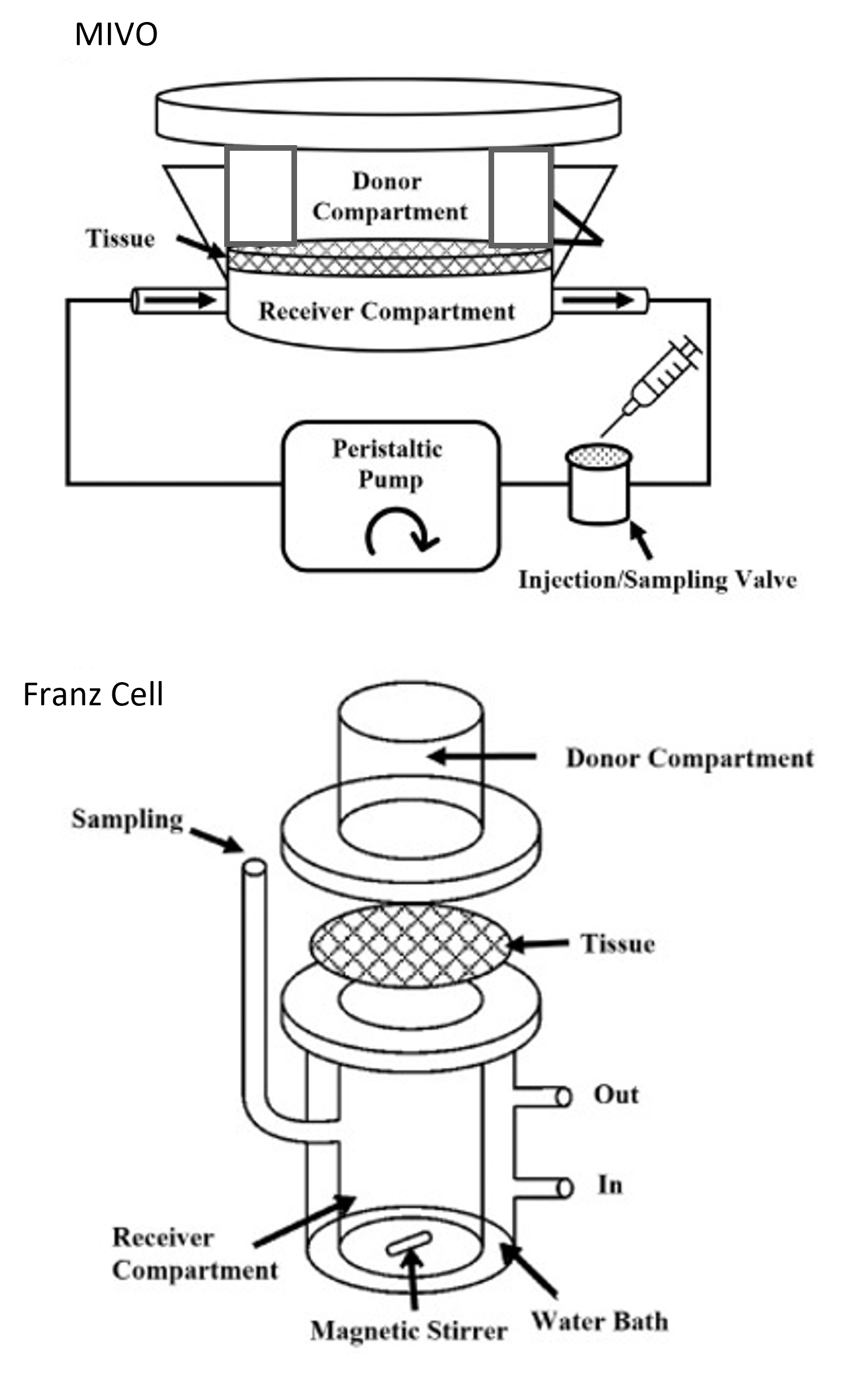
Our patented MIVO technology is a reliable alternative allowing to recreate both the three-dimensional complexity of the skin tissue and the microcirculation that feeds the tissue, affecting the absorption kinetics that characterize the real condition in vivo. On the one hand, our multi-chamber fluidic platform is suitable for culturing the classical skin cell monolayers, for hosting the commercially available 3D-reconstructed epithelial tissues (e.g. skin, vaginal wall, oral mucosa), as well as for the employment of skin biopsies or artificial membranes as skin surrogates. On the other hand, by connecting the MIVO with a peristaltic or syringe pump, it is possible to mimic the systemic circulation in vitro, in a highly controlled manner, by the application of a specific fluid flow rate according to the microenvironment that is desired to emulate (e.g., capillary, artery, vein…)..
The OECD428 guidelines “presents general principles for measuring dermal absorption and delivery of a test substance using excised skin”. Following these principles, the test substance “is applied to the surface of a skin sample separating the two chambers of a diffusion cell. The chemical remains on the skin for a specified time under specified conditions. The receptor fluid is sampled at time points throughout the experiment and analysed for the test chemical and/or metabolites”. Specific definitions of diffusion cell, receptor fluid, application to the skin, temperature conditions and sampling modalities are outlined, for the standardization of inter-laboratory protocols and improving the reproducibility of results.
In line with the OECD428 guidelines, the MIVO device consists in a donor and a receiver compartment, physically separated by the skin barrier only, where the skin can be cultured at the ALI (air-liquid interface). The test substance/medical device is applied on the apical side of the skin into the donor compartment, whereas the basolateral side is in contact with a laminar capillary flow, which (i) recapitulates the fluid-dynamic stimuli that physiologically affect the skin, increasing the relevance of the in vitro model, and (ii) receives the molecules that are absorbed through the skin. All these features, together with the efficient de-bubbling procedure, the fast and easy sampling, and the high reproducibility of the system, make the MIVO device an innovative tool for pharmacokinetic, cosmetic, toxicology and regulatory assays.

