Biomedical research is living one of the most exciting transitions in its history. For decades, scientists relied on 2D cell cultures and animal models to explore disease mechanisms and test new therapies. These approaches generated remarkable knowledge, yet they inevitably fall short when the goal is to truly recreate the biological complexity of human tissues. A flat monolayer of cells simply cannot reproduce the intricate architecture, biochemical gradients, and mechanical stimuli present in living organisms.
Today, 3D cell culture systems are rewriting the rules. By offering a spatially organized, physiologically relevant environment, they allow cells to behave more like they do inside the human body. This shift is not just a technical evolution: it is a conceptual leap forward. In cancer research, immunology, drug development, and personalized medicine, 3D cultures are revealing nuances that were invisible in traditional systems.
The Promise of 3D Cell Cultures: A More Realistic View of Human Tissues
Unlike 2D monolayers, 3D cell cultures reproduce key aspects of the native extracellular matrix (ECM), cell–cell interactions, and tissue dynamics. These models mimic the spatial organization of tissues and permit cellular behavior that closely reflects in vivo physiology, including proliferation, differentiation, migration, metabolic profiles, and treatment response. As a result, 3D systems significantly enhance translational accuracy, reducing the gap between in vitro observations and clinical outcomes.
Contemporary 3D culture systems encompass a wide range of biological configurations:
• Spheroids: Self-assembled aggregates that mimic the tissue or tumor microarchitecture, displaying physiologically relevant gradients of oxygen, nutrients, and therapeutic penetration.
• Organoids: Stem cell- or patient tissue-derived multicellular structures that recapitulate organ-specific architecture and function, enabling modeling of development and disease.
• Polymeric scaffolds: Engineered biomaterials providing ECM-mimicking support for organized tissue formation and mechanobiology studies.
• Bioprinted/reconstructed tissues: Layer-by-layer engineered constructs and differentiated cell models designed to replicate defined tissue structures.
• Tissue slices (ex-vivo explants): Thin sections of native tissues (e.g., tumor slices, liver slices) preserving natural stromal components, immune populations, and patient-specific microenvironments.
• Patient-derived biopsies: Directly obtained from clinical samples, representing the most authentic individualized disease models for precision medicine.
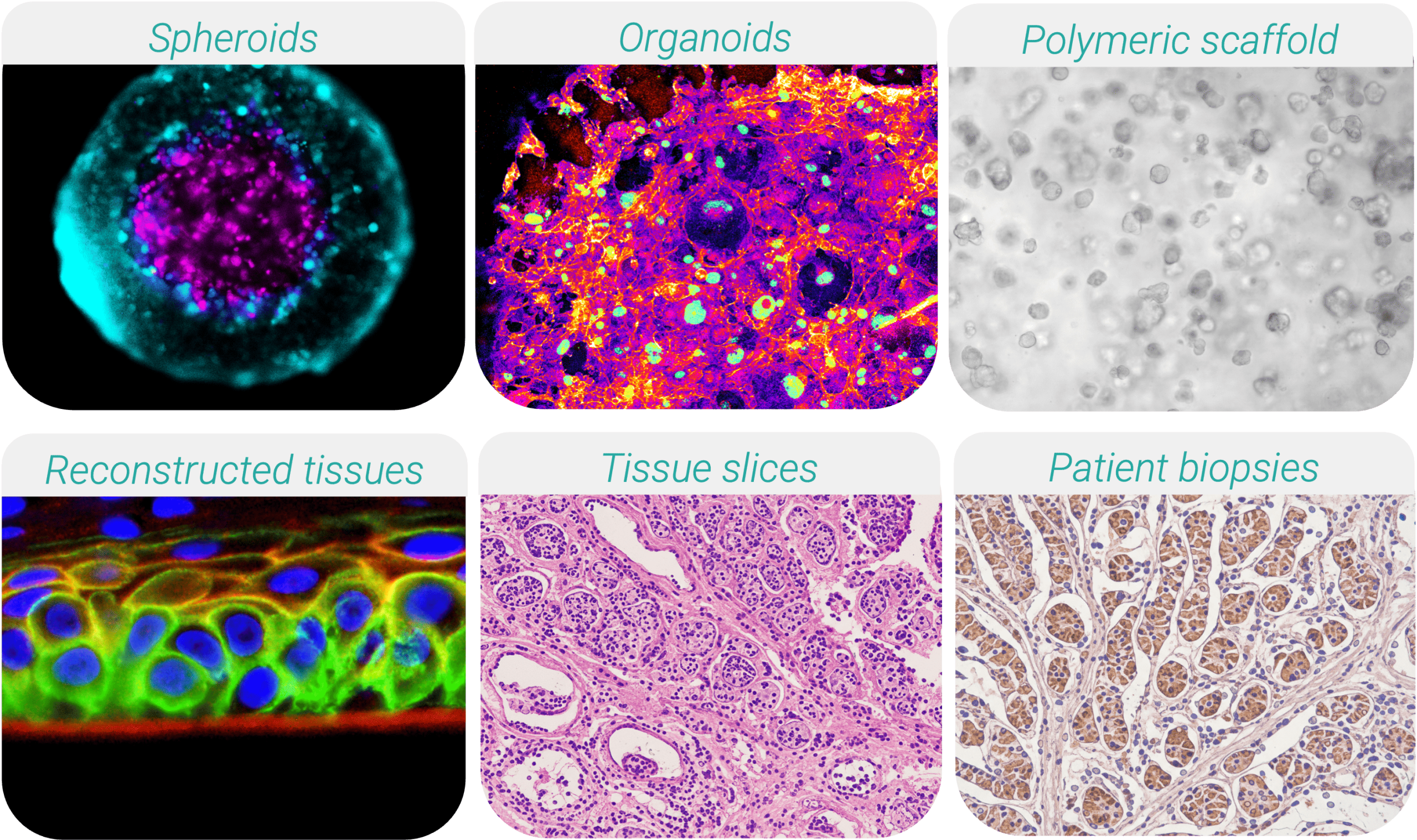
Among these platforms, tissue slices are gaining notable prominence. Their ability to preserve native extracellular matrix, vascular remnants, and immune infiltration makes them particularly valuable for immuno-oncology, drug transport studies, and tumor-stroma interaction assessments. However, maintaining viability and physiological perfusion ex vivo remains a core challenge, that dynamic culture technologies are uniquely positioned to solve.
The MIVO® Advantage in 3D Culture
The MIVO® platform represents a transformative advancement in 3D cell culture by integrating controlled physiological flow, which fundamentally enhances the physiological relevance and predictive accuracy of in vitro models. Unlike static 3D cultures, MIVO® dynamically replicates in vivo fluid dynamics, yielding critical advantages across multiple dimensions of experimental design and outcome.
- MIVO® dramatically extends tissue viability and functional organization. Flow conditions prolong tissue survival in vitro compared to static environments, enabling more robust long-term studies.
- MIVO® delivers superior predictive power for drug testing. The dynamic flow precisely replicates in vivo drug diffusion kinetics onto 3D tissues, producing efficacy readouts that align with physiological responses.
- MIVO® enables complex, multi-compartment modeling without specialized equipment. Its compatibility with standard 24-well plate inserts allows seamless conversion of static 3D protocols into dynamic systems, while its modular design supports co-culture with immune cells, microbs, or multi-organ configurations (e.g., intestine-liver connections) to simulate drug metabolism and tissue crosstalk. This capability unlocks chronic dosing, metastasis modeling (e.g., primary tumor to metastatic niche), and cell therapy testing—reducing reliance on costly in vivo models while improving reliability.
- MIVO®’s optical transparency and tray-based handling facilitate real-time monitoring of cellular dynamics under physiological flow, accelerating experimental throughput without additional instrumentation.
By bridging the gap between conventional static cultures and in vivo physiology, MIVO® delivers faster, more accurate, and cost-effective insights for oncology, drug permeability, and personalized medicine applications.

