Skin absorption assays represent a cornerstone in the development and evaluation of cosmetic formulations, transdermal drug delivery systems, and medical devices. These models provide critical insights into the permeation kinetics of active ingredients, directly informing bioavailability, efficacy, and safety assessments.
With increasingly stringent regulatory frameworks —driven by the full implementation of the EU Medical Device Regulation 2017/745 in 2021 and its recent amendments to reinforce product traceability, supply continuity, and EUDAMED activation, as well as ongoing compliance requirements under the EU Cosmetics Regulation 1223/2009—the need for physiologically relevant in vitro models has become increasingly pronounced.
In this context, a valuable scientific collaboration between Merck and React4Life has led to a peer-reviewed publication demonstrating the ability of MIVO® system to enhance prediction accuracy in dermal absorption studies. Leveraging this joint research effort, MIVO® technology introduces a paradigm shift in skin absorption testing, combining physiological relevance with compliance to OECD Test Guideline 428.
Overcoming Limitations of Franz Diffusion Cells
Traditional Franz Diffusion Cells (FDC) remain widely utilized, yet they inherently lack the ability to replicate the dynamic nature of human microcirculation. Fluid motion in FDC systems is dominated by rotational, non-physiological flow patterns, which can distort permeation behavior and limit predictive accuracy.
MIVO® technology fundamentally addresses these limitations by enabling laminar, monodirectional flow with spatially homogeneous velocity profiles consistent with capillary blood flow (~0.1 cm/s). This dynamic environment more accurately reflects the in vivo transport mechanisms that influence absorption, especially for lipophilic compounds, which tend to exhibit markedly different permeation profiles compared to hydrophilic molecules.
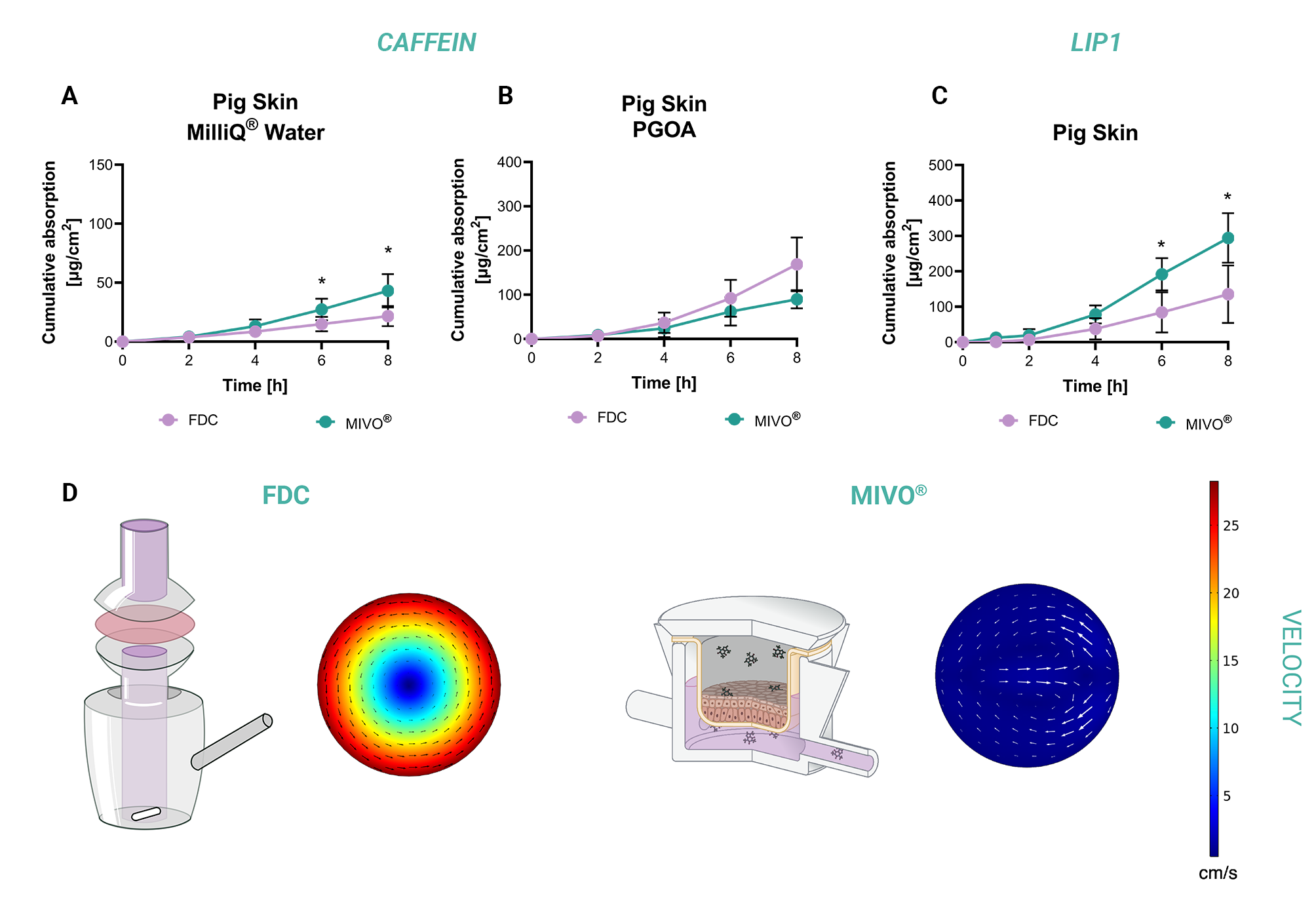
The significance of this physiological flow replication is demonstrated in this comparative study, showing the superior predictive accuracy of MIVO® over FDC in permeability testing. When caffeine (hydrophilic) and LIP1 (lipophilic, identical molecular weight) were evaluated using pig ear skin, both systems delivered comparable results for caffeine permeation. However, MIVO® revealed significantly greater penetration of the lipophilic molecule, aligning closely with established in vivo behavior. These results underscore the importance of replicating capillary-driven clearance to accurately model the kinetics of lipophilic substances.
Computational fluid dynamics (CFD) simulations further substantiate the advantages of MIVO®. The simulations revealed that MIVO® generates a controlled, spatially homogeneous velocity profile below the skin, closely matching physiological capillary blood flow conditions. In contrast, FDC exhibited a non-physiological rotational flow pattern with velocities ranging from arterial to venous values in different regions of the receptor compartment. This undesirable vortex formation disrupts the static fluid layer adjacent to the membrane, altering the boundary layer conditions that are fundamental to permeation governed by Fick’s law, compromising data reliability.
Designed for Versatility and Operational Efficiency
MIVO’s technological advantages extend beyond improved physiological relevance. The device features an integrated three-way valve system for efficient, non-invasive sampling of receptor fluid without compromising sterility or tissue integrity. This capability, combined with the device’s compatibility with cell culture incubators (32°C, 5% CO₂), allows for extended testing periods with minimal experimental disruption. The system’s design also accommodates a variety of skin models including artificial membranes (Strat-M®), 3D-reconstructed epithelial tissues, and skin biopsies, making it versatile for multiple applications.
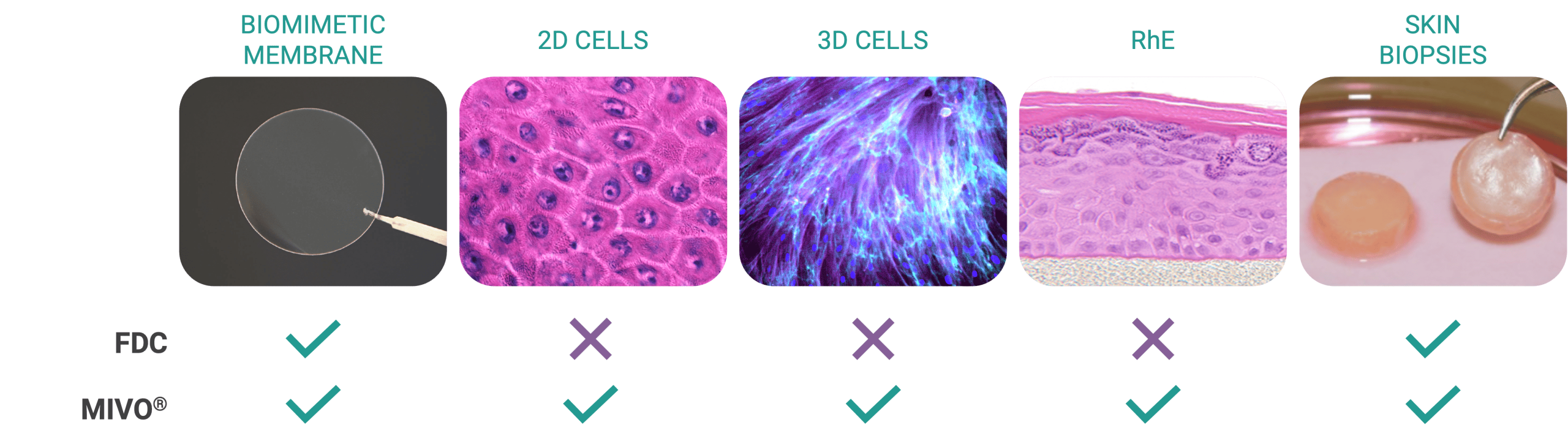
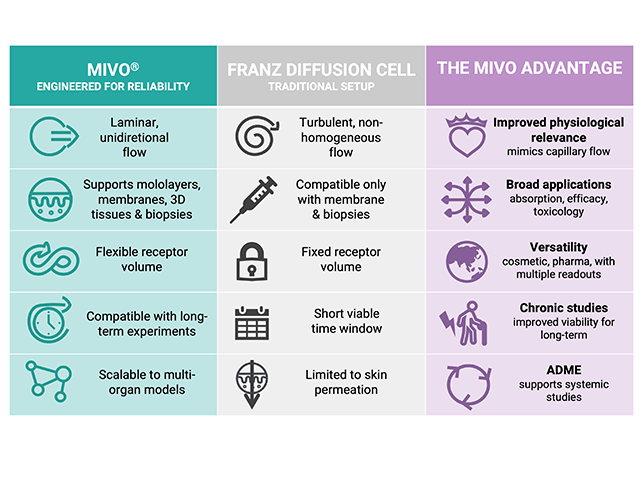
Importantly, MIVO maintains full compliance with OECD 428 guidelines for in vitro skin absorption testing. The device consists of a donor compartment and a receiver compartment separated by the skin barrier, with the skin cultured at the air-liquid interface (ALI) – precisely matching the OECD 428 standard. The MIVO system’s ability to simulate physiological fluid dynamics while adhering to established regulatory frameworks creates a powerful bridge between traditional in vitro testing and in vivo relevance.
For cosmetic and medical device developers, MIVO® technology offers several strategic advantages. The improved prediction accuracy for lipophilic compounds – which often constitute a significant portion of active ingredients in cosmetic formulations – enables more reliable formulation development and risk assessment. The high reproducibility of MIVO® results reduces inter-laboratory variability, addressing a key limitation of traditional diffusion cell methods. Furthermore, the system’s compatibility with the 3Rs (reduce, replace, refine) approach to animal testing makes it an attractive alternative for ethical research.
The implications extend beyond product development to regulatory submissions. As regulatory bodies increasingly demand physiologically relevant data to support classification decisions under EU Regulation 2017/745, MIVO®’s ability to replicate human skin microcirculation provides a compelling scientific basis for classifying medical devices as Class IIa (local absorption) versus Class III (systemic absorption). This distinction has significant implications for product development timelines, testing requirements, and market access.
In conclusion, MIVO® technology represents a significant advancement in skin absorption testing, moving beyond the limitations of conventional diffusion cells toward true physiological modeling.
For the cosmetic industry, medical device manufacturers, and pharmaceutical companies, MIVO® provides not just a more accurate testing platform, but a strategic tool for accelerating product development, ensuring regulatory compliance, and ultimately delivering safer, more effective products to consumers.

