In modern oncology, cancer is no longer viewed as a single disease but rather as a vast constellation of molecularly distinct disorders. Each tumor arises from a unique combination of genetic mutations and cellular behaviors, making the traditional “one-size-fits-all” therapeutic paradigm increasingly obsolete.
This understanding has propelled the field toward personalized medicine, where treatments are tailored to the biological and genetic profile of each patient.
While patient‑derived organoids and tissue slides have emerged as powerful disease models, their predictive capacity is limited by the absence of a physiologically relevant microenvironment. The MIVO® Organ‑on‑Chip platform bridges this critical gap by integrating organoid biology with dynamic fluidic engineering, thereby enabling a truly personalized approach to cancer therapy.
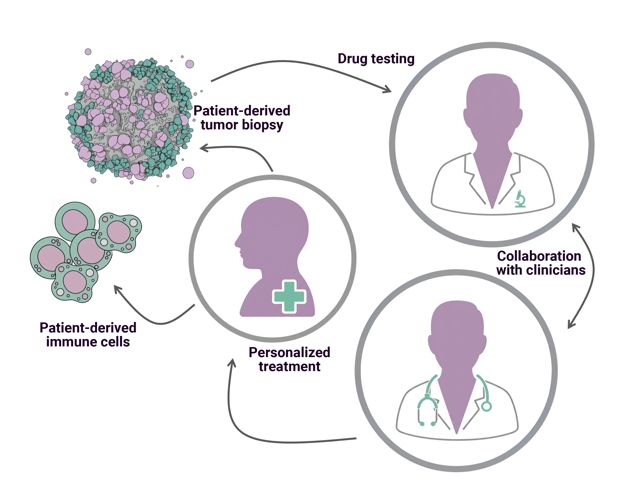
Organoids and tissue slices: a foundation for personalized medicine.
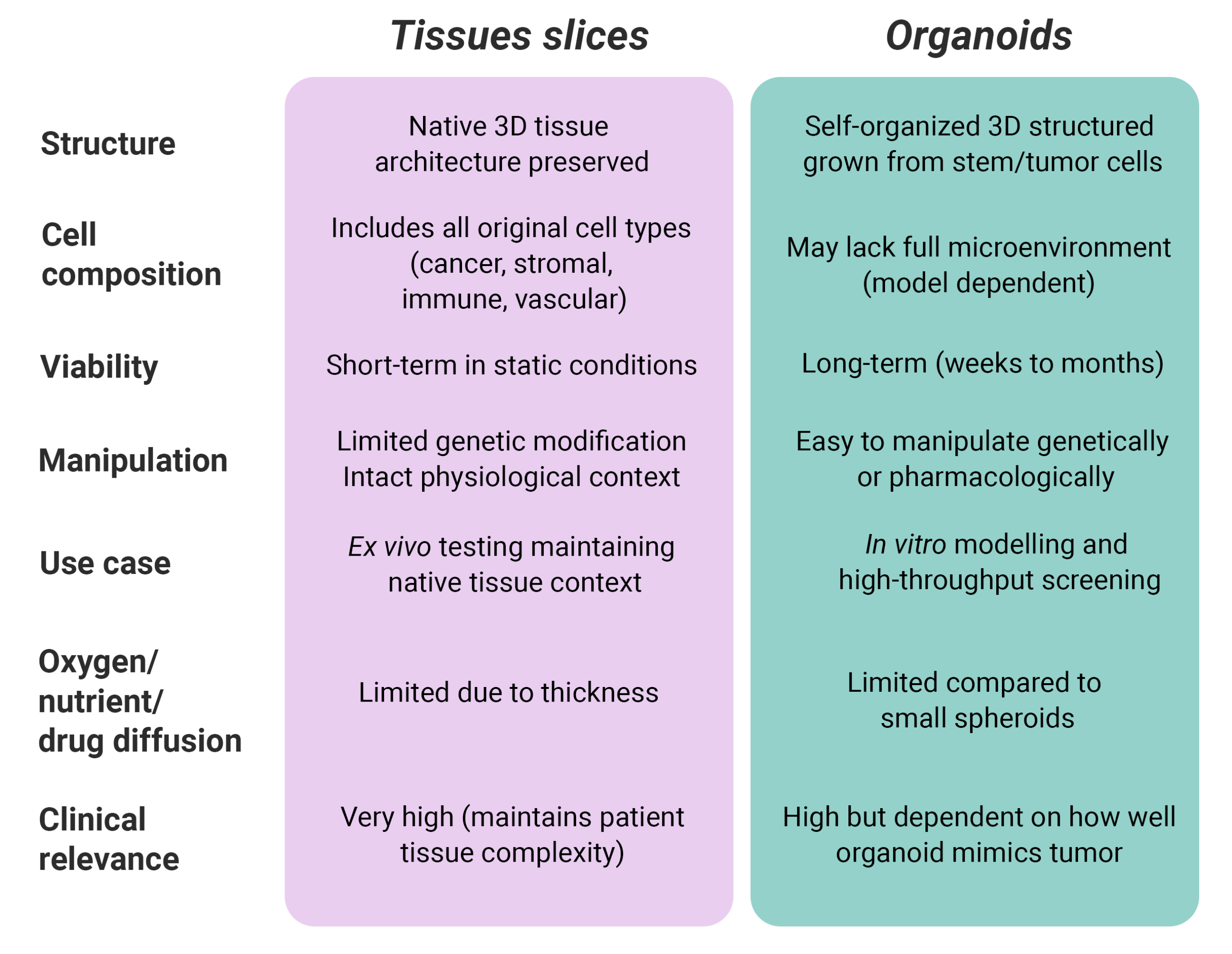
Organoids are three-dimensional structures generated from patient-derived stem or tumor cells that self-organize into miniature versions of human tissues. They preserve the genetic, phenotypic, and functional characteristics of the original tumor, offering an unprecedented level of biological relevance compared to conventional models.
Similarly, tissue slices or tissue explants are viable sections of tissue that preserve the native 3D architecture and cellular composition of the tumor microenvironment, therefore very useful for functional studies.
Unlike two‑dimensional cultures, they indeed recapitulate the architectural complexity of tumors, including stromal‑epithelial interfaces and extracellular matrix organization. However, despite their sophistication, there 3D tumor models lack one key feature: the ability to reproduce the dynamic microenvironment of the human body, including mechanical forces, nutrient gradients, and immune interactions that profoundly influence drug response.
MIVO®: recreating the human microenvironment in vitro
MIVO® technology provides a revolutionary solution by introducing a controlled, fluid-dynamic environment that emulates the physiological conditions found in vivo. It precisely replicates the biochemical and mechanical stimuli of human tissues, including blood flow dynamics that affect drug delivery, cellular communication, and immune activation.
Moreover, it perfectly resembles the dynamic diffusion of soluble molecules into 3D tumor models, for more reliable readouts.
By enabling the co-culture of patient-derived 3D tumor models with a physiological, controlled flow resembling the bloodstream, MIVO® generates a more physiologically relevant and predictive model of tumor biology.
The platform’s versatility extends to multi‑organ configurations, allowing the study of drug distribution, metabolism, and organ‑specific toxicity in a single, integrated system. For instance, by coupling a primary tumor compartment with downstream “metastatic niche” modules, such as liver, bone marrow, or brain tissue, MIVO® can reproduce metastatic dissemination and therapeutic response in a physiologically faithful context.
Simulating systemic drug administration and immune dynamics
One of MIVO®’s most compelling advantages is its ability to simulate systemic drug administration. Therapeutic agents can be delivered through the dynamic flow at clinically relevant concentrations and flow rates, mirroring intravenous exposure.
This feature is especially pivotal for immuno-oncology, where the dynamic interplay between circulating immune effectors (e.g., CAR‑T cells, NK cells) and tumor cells determines clinical outcomes.
MIVO® enables real‑time observation of immune cell migration, extravasation, and tumor‑cell killing under physiological flow conditions that replicate in‑vivo shear stress. This provides unprecedented insight into immune-cell infiltration dynamics and the cytotoxic efficacy of immunotherapies. Researchers can thus evaluate therapeutic potency and safety before clinical administration, enabling more informed and patient-specific treatment design.
This technology offers several distinct advantages over conventional approaches:
• Enhanced Predictive Accuracy: MIVO®‘s fluid-dynamic environment more faithfully replicates the tumor microenvironment, esulting in drug-response data that correlate more closely with clinical outcomes.
• Personalized Treatment Selection: Multiple therapeutic options can be tested on patient-derived organoids within MIVO®, allowing clinicians to identify the most effective treatment for an individual patient before initiating therapy
• Reduced Reliance on Animal Models: MIVO®offers a human-relevant, ethical alternative to xenograft models, improving translational relevance while reducing animal use.
• Comprehensive Drug Testing: The platform supports parallel assessment of efficacy, toxicity, and pharmacokinetics across interconnected tissue models.
• Dynamic Immune Interaction Studies: MIVO® enables controlled, high-fidelity studies of immune cell migration, extravasation, and tumor targeting—essential for advancing next-generation immunotherapies.
Toward Truly Personalized Cancer Therapy
Integrating MIVO® into cancer research represents a paradigm shift in how we model disease and design therapies. By merging biological realism with experimental precision, MIVO® bridges the long-standing gap between static in vitro assays and complex in vivo systems.
Incorporating MIVO® into the drug-development pipeline transforms the traditional linear workflow into a dynamic, patient-centric process, representing therefore a transformative step toward truly personalized cancer therapy.
As research continues to validate its predictive potential, the MIVO® platform stands poised to become a cornerstone of precision oncology, driving the transition from empirical treatment selection to evidence-based, individualized therapy, ultimately improving outcomes while minimizing toxicity and cost

