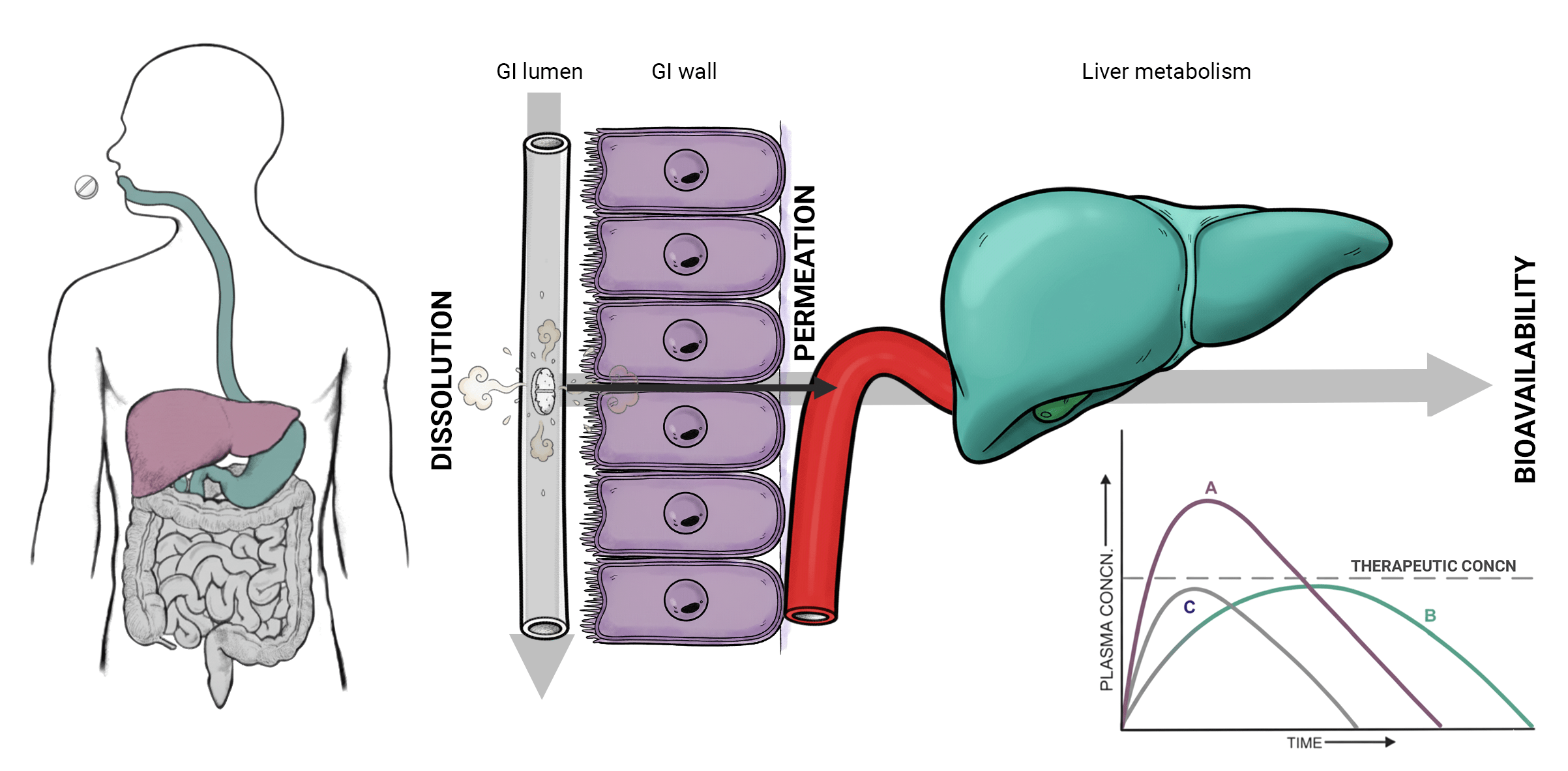
Oral drug administration remains the most widely adopted administration route for therapeutic delivery worldwide. Tablets, capsules and other solid dosage forms are indeed convenient, stable, cost-effective and generally accepted by patients. However, this apparent simplicity conceals substantial scientific complexity. To be clinically effective, an orally administered drug must first dissolve in gastrointestinal fluids and then successfully cross the intestinal barrier before entering systemic circulation. These two sequential but intimately interconnected processes, named dissolution and permeation, respectively, together define the fraction of the dose absorbed (Fabs). This fraction is a direct measure of the drug’s absorption efficiency and a primary determinant of oral bioavailability.
Bioavailability, in essence, expresses the amount of administered active pharmaceutical ingredient (API) able to reach systemic circulation at therapeutically effective concentrations. If dissolution is insufficient or slow, drug molecules may never become available for absorption. Conversely, even a fully dissolved compound may fail to exert therapeutic action if it does not permeate the intestinal epithelium efficiently.
For decades, pharmaceutical scientists have studied dissolution and permeation as separate events, often in different experimental systems, and this separation has imposed significant limitations on our ability to accurately predict in vivo absorption profiles and in vitro–in vivo correlations (IVIVC).
Today, the paradigm is shifting. The scientific community increasingly recognizes that treating dissolution and permeation as interconnected phenomena, and not as isolated steps, is essential to build a more realistic and predictive understanding of oral drug performance. This evolving mindset sets the stage for cutting-edge technologies capable of replicating physiological processes more faithfully and efficiently.
MIVO® as a Simultaneous Dissolution-Permeation Platform
A fruitful collaboration with Prof. Di Cagno and his research group at the University of Oslo has recently led to a significant advancement in this field. Their joint effort culminated in a publication showcasing a pioneering approach: performing simultaneous dissolution and permeation studies within a single dynamic platform, enabled by the MIVO® system. While conceptually intuitive, this achievement required a sophisticated model able to track drug dissolution in real time while accurately reproducing the transport barrier mimicking the intestinal epithelium.
The study employed the MIVO® system in its Meso-fluidic Chip for Permeability Assessment (MCPA) configuration, which integrates the MIVO® cell-culture chamber with the PermeaPad biomimetic membrane. The platform was also coupled with automated UV/Vis spectrophotometry via flow-through cuvettes, streamlining analytical workflow while improving reproducibility and minimizing operator-related variability.
Crucially, the study confirmed that the MIVO® system is capable of capturing subtle differences in drug behavior, both molecule- and formulation-dependent. The technology proved versatile across compounds with markedly distinct permeability characteristics, ranging from highly permeable caffeine to low-permeability ibuprofen, and was sensitive enough to detect formulation-driven effects on permeation performance. As a notable example, hydrocortisone amorphous solid dispersions (ASDs) exhibited roughly twice the permeation rate of their crystalline counterparts, highlighting the platform’s ability to quantitatively discriminate enhanced bioavailability linked to formulation design.
This study represented a proof of concept, demonstrating how real-time monitoring of both dissolution and permeation in the same experimental setup can offer deep mechanistic insights into absorption behavior. By observing drug dissolution and membrane transport concurrently, researchers could directly visualize and quantify the interplay between solubilization kinetics and barrier transport, something that traditional, sequential testing approaches simply cannot capture with comparable fidelity.
This capacity does not merely accelerate experimentation, but reshapes the scientific narrative. Instead of treating in vitro data as fragmented snapshots of independent processes, MIVO® enables a continuous, physiologically relevant absorption story to emerge. And this, in pharmaceutical science, is transformative.
From standard models to biopsies, immune cells and microbiota: building human-relevant biology inside MIVO®
Being conceived as a human-centric platform, one of the MIVO®’s key strengths is the biological versatility and full compatibility with several tissue models. Although the described study employed a synthetic membrane as a functional intestinal barrier surrogate, the system can be populated with immortalized cell monolayers, differentiated human enterocyte cultures, primary tissues, intestinal organoids, and even human biopsies when available. This flexibility makes MIVO® uniquely capable of evolving alongside the biological question under investigation. Researchers may begin with a simplified barrier to screen basic permeation behavior and then progressively increase biological complexity, introducing mucus-producing co-cultures, immune-competent environments, or microbial communities when required.
An especially compelling advantage is the possibility to incorporate immune cells, commensal bacteria or pathogenic strains, depending on the research focus. This ability to enrich the gastrointestinal microenvironment with relevant cellular and microbial actors means that MIVO® can go beyond passive diffusion modeling and begin to explore how mucosal immunology, microbial metabolism and inflammatory pathways influence drug uptake. This direction is particularly relevant for drugs whose bioavailability is shaped by host–microbiome interactions or disease-driven barrier alterations, where conventional permeability assays fail to capture reality.
Recreating the Entire Oral Journey: Digestion, Absorption and Metabolic Fate
A further dimension sets MIVO® apart from conventional in vitro dissolution and permeation tools: its ability to evolve into a multi-compartment, physiologically guided model of the entire oral drug journey. Beyond intestinal absorption, MIVO® can be configured to recapitulate upstream digestive stages, bringing dissolution, digestion, and epithelial transport into a single continuous experimental flow.
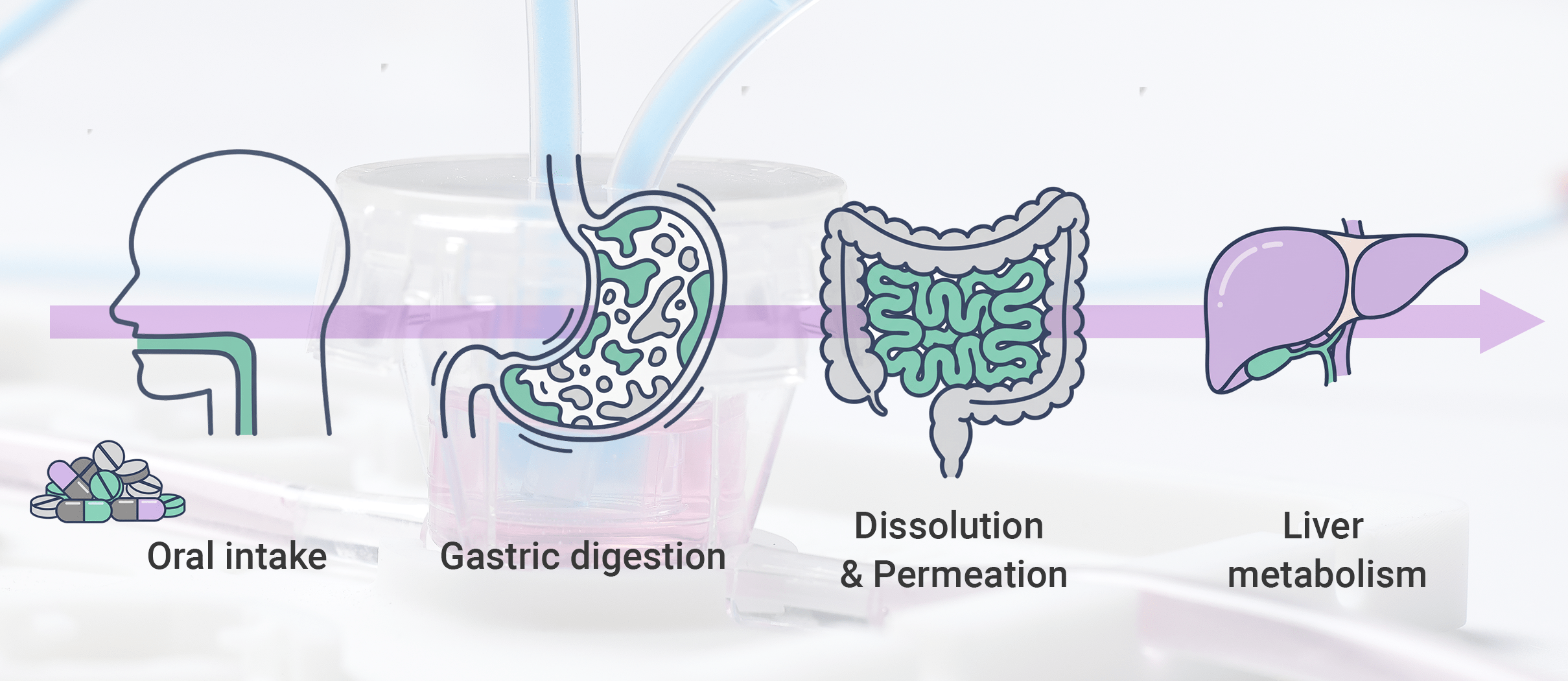
Through modular integration, the platform can emulate oral, gastric, and intestinal environments, capturing key physiological drivers such as pH transitions, enzymatic hydrolysis, bile interactions, residence times, and mechanical agitation. This enables following a formulation from the moment of “administration” through its biochemical transformation and solubilization, ultimately reaching a biologically relevant absorption interface.
While conceptually aligned with state-of-the-art gastrointestinal simulation frameworks, the MIVO® ecosystem introduces a decisive advantage: the output of digestion does not terminate as a theoretical endpoint. Instead, digested material seamlessly transitions into a dynamic absorption chamber, where epithelial transport is evaluated under realistic conditions. Dissolution is therefore no longer isolated from digestion, but evaluated as part of the real cascade that governs oral bioavailability.
Beyond the intestinal barrier, MIVO®’s multi-organ connectivity enables downstream coupling to liver modules to reproduce first-pass metabolism. The result is a physiologically coherent sequence — digestion, epithelial uptake, hepatic processing — that approaches a mechanistic reconstruction of true bioavailability.
In an era where mechanistic rigor and translational relevance drive regulatory and industrial expectations, this capability marks a paradigm shift. Systems that evaluate dissolution or permeability in isolation are increasingly limited in their ability to predict clinical outcomes. The future of oral drug assessment lies in models that respect biological complexity without sacrificing experimental control.
MIVO® embodies this evolution, being a modular, human-relevant physiological ecosystem capable of expanding with scientific ambition, from simple permeability screening to integrated digestion-absorption-metabolism pipelines.
Reference:
1Tzanova MM, Larsen BS, Birolo R, Cignolini S, Tho I, Chierotti MR, Perissutti B, Scaglione S, Stein PC, Hiorth M, Di Cagno MP. Shifting the Focus from Dissolution to Permeation: Introducing the Meso-fluidic Chip for Permeability Assessment (MCPA). J Pharm Sci. 2023 Dec 16:S0022-3549(23)00538-5.

