Understanding how orally administered compounds traverse the human intestinal barrier is a cornerstone of modern drug development. Yet, accurately replicating the physiological processes governing intestinal permeability has long represented a major scientific challenge. Conventional in vitro systems—typically based on static monolayers such as Caco-2 cells—lack the cellular diversity, architectural complexity, and dynamic microenvironment characteristic of native human intestinal tissue. As a result, these models frequently fail to reproduce human-relevant absorption kinetics, limiting their predictive value for pharmaceutical research.
Against this backdrop, MIVO® (Multifunctional In-Vitro Organ) technology has emerged as a transformative fluid-dynamic platform capable of reproducing the structural and functional features of the small intestine with unprecedented fidelity. By integrating 3D reconstructed human intestinal tissue (EpiIntestinal™) with a physiologically inspired perfusion system, MIVO® provides a powerful alternative to static assays and animal studies, enabling controlled, quantitative investigations of both transcellular and paracellular absorption mechanisms.
The physiology of intestinal permeability
The human intestinal barrier represents one of the most complex and dynamic physiological systems in the body, acting as a selective gatekeeper between the external environment and the internal milieu. This specialized tissue, composed of various cell types including enterocytes, goblet cells, and enteroendocrine cells, maintains a delicate balance between nutrient absorption and pathogen exclusion.
Two parallel pathways mediate this transport:
- Transcellular absorption, in which molecules traverse the epithelial cell membrane.
- Paracellular absorption, regulated by tight junction proteins that control solute passage through intercellular spaces.
Dysregulation of this barrier, a phenomenon known as “leaky gut”, is associated with several gastrointestinal and systemic conditions, including inflammatory bowel disease, celiac disease, small-intestinal bacterial overgrowth, and metabolic disorders. Clinically, intestinal permeability is often assessed using the lactulose/mannitol ratio (LMR), a well-established metric that distinguishes alterations in the paracellular route (lactulose) from changes in transcellular transport (mannitol).
Replicating these mechanisms in vitro, however, has been historically difficult. Static systems lack appropriate villus architecture, produce non-physiological tight-junction profiles, and cannot reproduce the fluid dynamics that drive nutrient exchange and waste removal in vivo. This is precisely where MIVO® introduces a paradigm shift.
MIVO®: human-relevant platform for predictive gut absorption
MIVO® technology has emerged as a transformative approach to studying intestinal permeability, offering a sophisticated fluidic 3D in vitro model that accurately replicates the physiological conditions of the small intestine. Unlike traditional static cell culture models, MIVO® incorporates dynamic fluid flow and mechanical stimulation that mimic the natural environment of the intestinal tract, providing unprecedented insights into molecular absorption pathways.
By integrating EpiIntestinal™, a 3D human tissue, with a closed-loop fluid dynamic system, MIVO® creates an environment that closely mirrors the mechanical, biochemical, and structural stimuli of the native small intestine. The platform supports physiological circulation below the epithelial membrane, mimicking capillary flow and continuously supplying nutrients while removing metabolic byproducts, conditions that static cultures simply cannot reproduce.
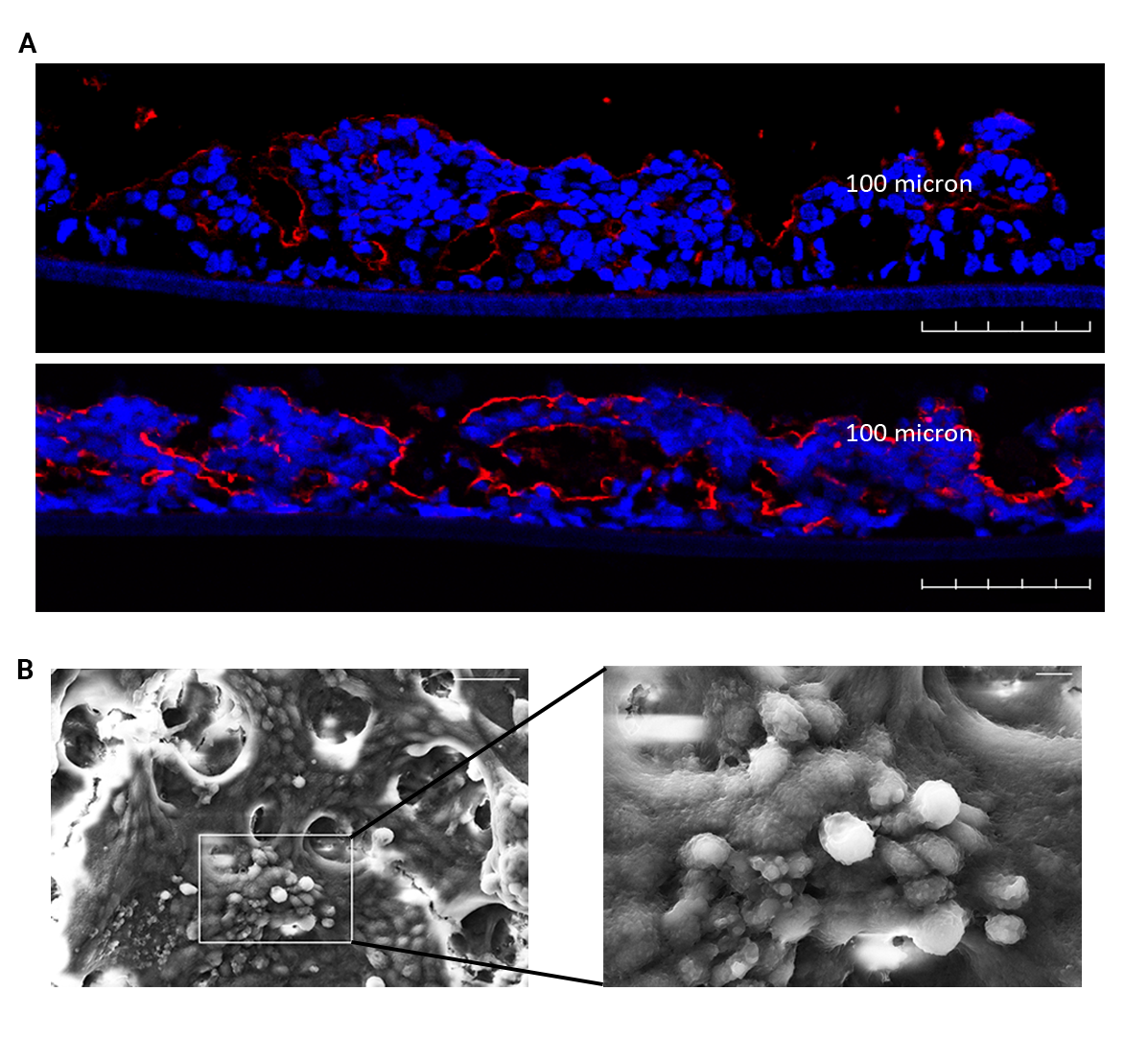
The benefits of this dynamic environment become evident when examining the morphology of tissues cultured within the device. Under MIVO® flow, villin expression at the brush border becomes more pronounced, microvilli develop robustly, and overall epithelial architecture appears healthier and more physiologically aligned with native tissue than in static controls. These structural differences translate directly into more accurate functional behavior, particularly in permeability studies.
Quantitative assessment of paracellular and transcellular intestinal absorption
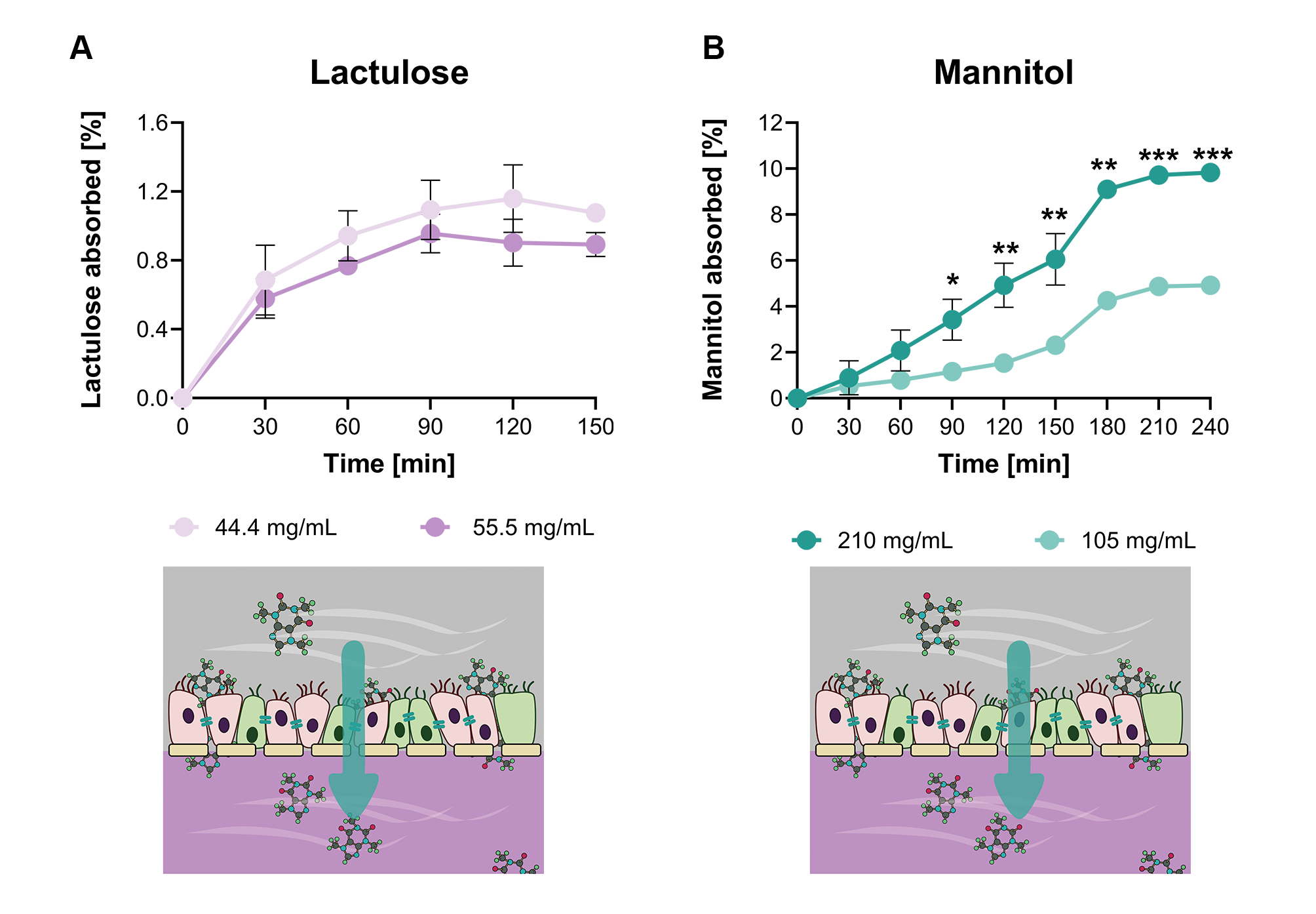
To demonstrate the platform’s capacity to discriminate between the two major intestinal absorption routes, the study applied the well-established lactulose/mannitol assay, a clinical standard for evaluating epithelial integrity and detecting conditions commonly referred to as “leaky gut.” Mannitol, a small monosaccharide that crosses the epithelium primarily through transcellular pathways, consistently exhibited higher permeation than lactulose, a larger disaccharide whose movement is restricted to the paracellular route and therefore closely tied to tight-junction integrity. Under healthy dynamic conditions in the MIVO® system, both molecules reached a characteristic steady-state absorption plateau, and the calculated lactulose/mannitol ratio aligned with values typically observed in healthy human subjects, underscoring the physiological relevance of the MIVO® platform.
Modelling leaky gut and intestinal barrier function recovery
A particularly compelling demonstration of MIVO®’s capabilities emerged when modeling barrier dysfunction. Tight-junction disruption was induced using EGTA, a calcium chelator widely employed to mimic pathological increases in paracellular permeability. As expected, lactulose transport increased markedly, reflecting the kind of paracellular leakiness associated with inflammatory or immune-mediated intestinal disorders. Mannitol absorption, on the other hand, remained largely unchanged, consistent with its reliance on transcellular pathways. This differential behavior not only confirmed the sensitivity of the model but also mirrored clinical patterns characteristic of compromised epithelial barriers.
However, the most striking insight came from observing how the tissue recovered after injury. When EGTA-treated tissues were returned to normal culture conditions, only those maintained under MIVO®’s dynamic flow regained physiological levels of lactulose permeability. In static cultures, the barrier remained persistently leaky, failing to restore tight-junction integrity despite identical exposure to repair-promoting media. This discrepancy highlights the critical role of fluid dynamics in epithelial healing: dynamic flow improves removal of waste products such as lactate, prevents local acidification, enhances nutrient distribution, and supports the natural turnover of intestinal cells. Through these mechanisms, MIVO® recreates the microenvironment essential not only for maintaining barrier integrity but also for restoring it following injury.
Why MIVO® matters for pharmaceutical and nutraceutical research
With its ability to reproduce in vivo-like permeability patterns, MIVO® provides a reliable platform for:
- Predicting oral drug absorption during early discovery phases.
- Reducing dependence on animal studies in line with 3R (Reduce, Refine, Replace) principles.
- Screening compounds for epithelial toxicity and barrier-modulating effects.
- Elucidating disease mechanisms underlying leaky-gut-associated disorders.
- Evaluating functional foods and nutraceuticals impacting gut barrier integrity.
By enabling precise, mechanistic, and quantitative analyses of epithelial transport routes, MIVO® bridges a long-standing gap between simplistic static models and complex in vivo systems.

