The development of new research methods for drug analysis represents one of the most significant aspects of modern drug development. However, this complicated process still faces challenges including time consumption, low-throughput, poor reliability, and high costs.
Traditional 2D static cultures often fail to predict in vivo responses due to their intrinsic inability to properly mimic the tissue physiological architecture. This gap has driven the development of novel in vitro systems, including microphysiological systems (MPS) like MIVO®. The core concept is to provide a fluid-dynamic cell culture environment that better resembles the human body, enhancing drug discovery and disease understanding.
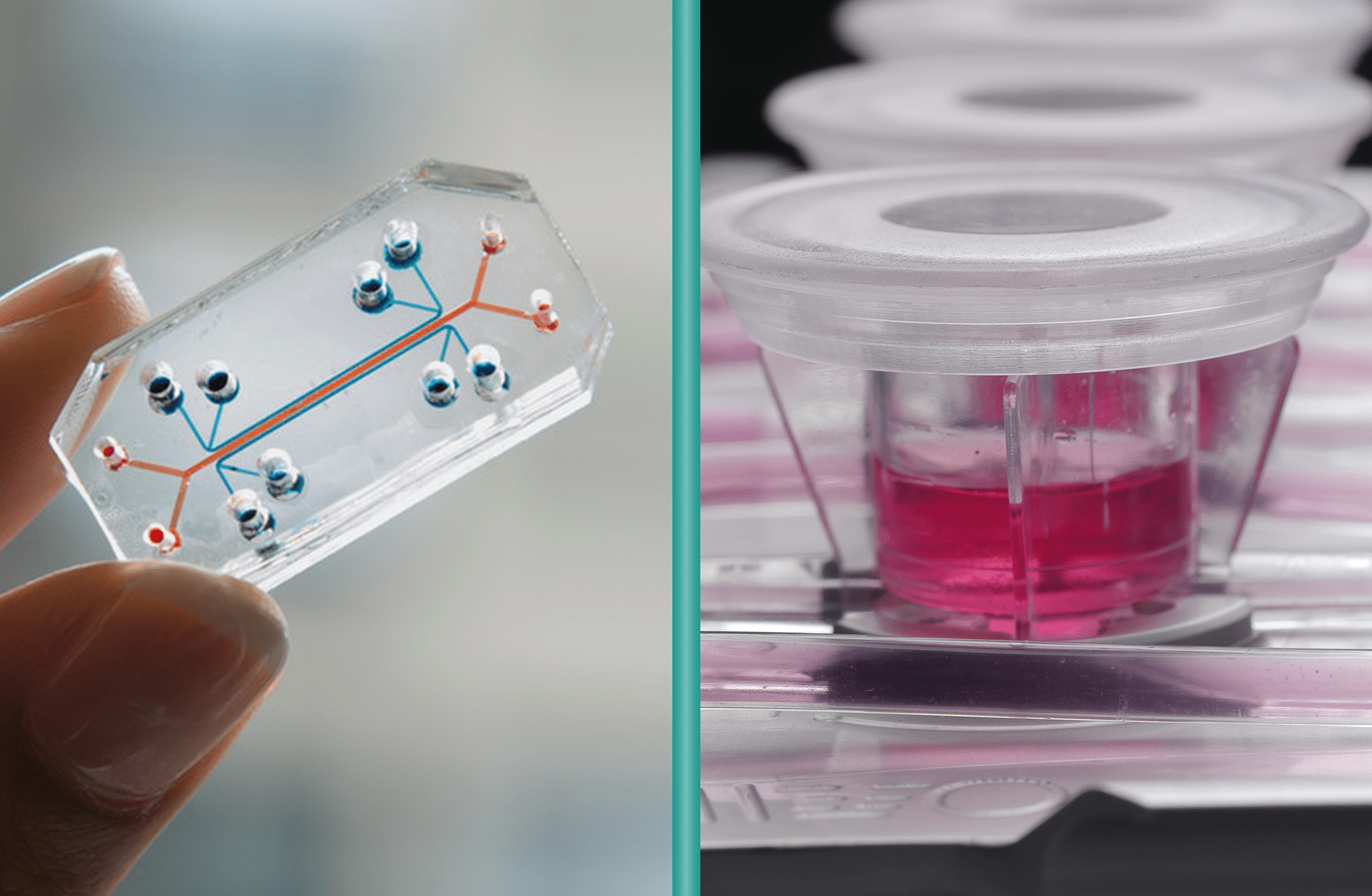
Microfluidic “lab-on-a-chip” platforms have gained popularity for high-throughput drug screening, but they come with important limitations for broader drug development studies. These tiny systems condense multiple laboratory functions onto a chip just a few centimeters across, with internal channels measuring only few micrometers. This extreme miniaturization drastically reduces sample requirements, allowing experiments at the single-cell level. However, this comes with a trade-off: the small scale can compromise the physiological relevance of the results.
The Critical Difference: Scale and Physiological Relevance
The key distinction between MIVO® and conventional microfluidic organ-on-chip systems lies in scale and physiological fidelity. Microfluidic chips typically accommodate only a few hundred cells, whereas MIVO® has been designed to host clinically relevant size 3D tissue models including patient-derived biopsies, bioprinted scaffolds, and commercialized reconstructed tissues.
MIVO® reproduces the mechanical shear stress typical of in vivo fluid dynamics, simulating the dynamic flow of blood capillaries that nourish tissues and transport nutrients and drugs. This is made possible by handling larger fluid volumes—up to several milliliters—at controlled flow rates, enabling the platform to support both small-scale tissue models and larger, clinically relevant constructs.
Why MIVO® Outperforms Microfluidic Organ-on-Chip Systems
• Physiological Scale: MIVO® is the only organ-on-chip technology that combines clinically relevant tissue size with independent millifluidic environments where fluid flow is fully controlled. This enables the study of tissue behavior under conditions that closely mirror human physiology.
• Enhanced Functional Assessment: Microfluidic organ-on-chip devices often fail to fully exhibit the authentic functionalities of organs due to their small size. MIVO® overcomes this limitation, allowing researchers to observe complex tissue behaviors that would be impossible in miniature systems.
• Multi-Organ Integration: While microfluidic chips typically handle single-organ models, MIVO® allows for fluidically connected multi-organ configurations where different tissue types share the same simulated blood flow. This enables comprehensive studies of organ-organ interactions under physiological flow conditions.
• Practical Application: The smaller scale of microfluidic chips makes them unsuitable for late-stage drug development and preclinical studies requiring complex, in vivo-like environments. MIVO® bridges this gap, providing a platform that can effectively investigate drug toxicity and efficacy in a more relevant context.
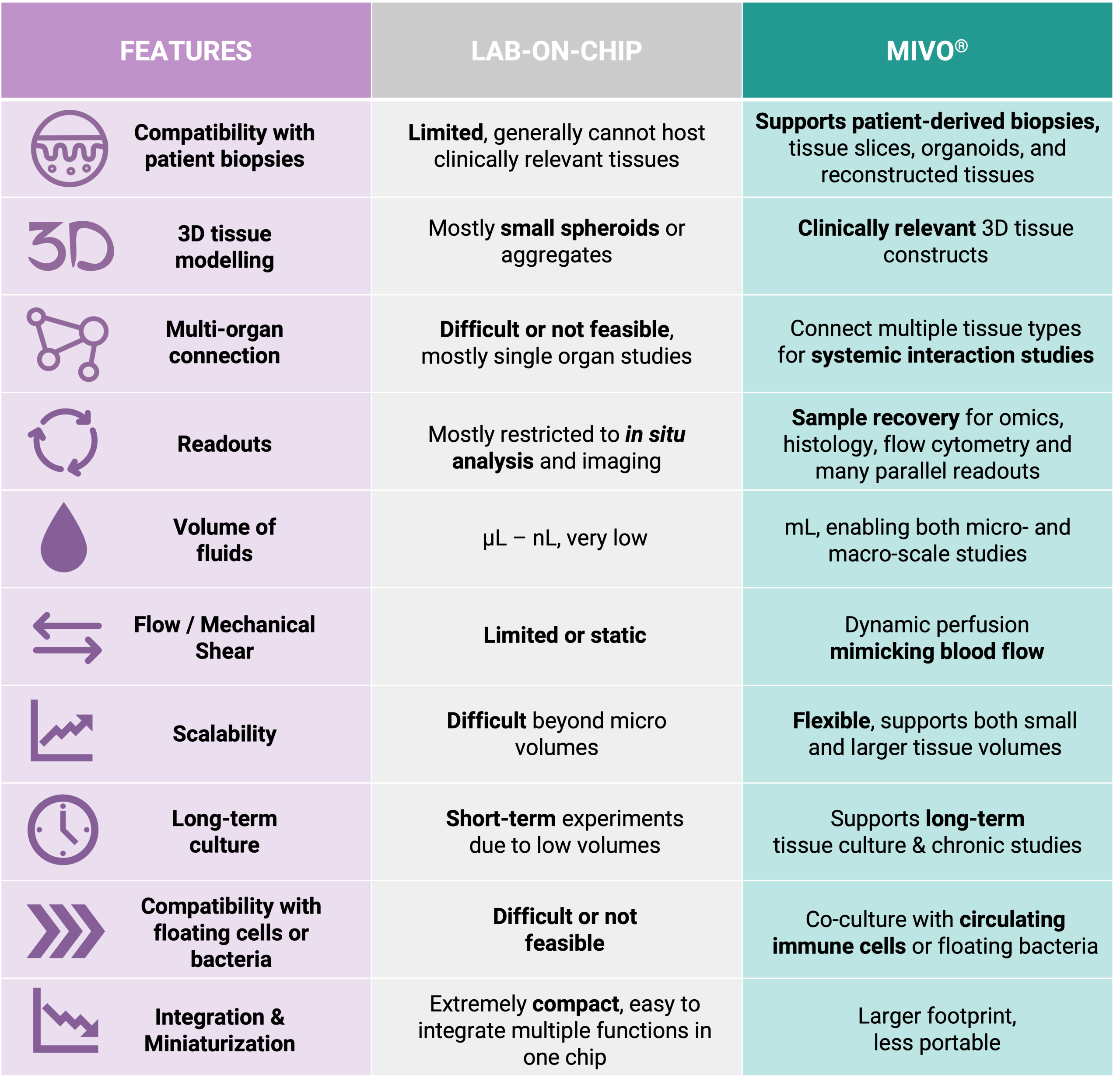
By addressing the fundamental limitation of scale in microfluidic organ-on-chip systems, MIVO® represents a significant advancement in creating predictive in vitro models that better translate to clinical outcomes. This technology enables researchers to move beyond simplistic 2D cultures and microfluidic miniaturization toward truly human-relevant drug testing platforms.
With MIVO®, the future of drug development is no longer constrained by the limitations of miniature systems but instead empowered by the ability to recreate human physiology at the scale that matters.

