MIVO® technology has made possible the implementation of a fluidic 3D in vitro model of the intestinal barrier. In this article it is demonstrated that MIVO®, combined with a 3D intestinal epithelium model, allows to emulate the intestinal environment in vitro, as a reliable platform for the study of the passage of molecules, discriminating the two main intestinal absorption mechanisms. This platform therefore can be employed for the quantification of the absorption of oral drugs.
The intestinal epithelium has a well-organized cellular structure, with different cellular phenotypes and barrier properties that make it effective against the excessive absorption of food antigens. When this mechanism doesn’t work properly, the antigens pass into the bloodstream in big quantities, causing, in some individuals, a responsiveness of the immune system. The increase in intestinal permeability leads to a passive absorption of substances that are normally excluded, triggering a multitude of clinical disorders. Intestinal permeability is therefore crucial in regulating the bioavailability and consecutively the biological effects of drugs and food compounds. However, systematic and quantitative studies on the absorption of molecules are quite limited, due to the lack of reliable experimental models capable of reproducing the responses observed in vivo.
MIVO® technology can provide an innovative platform that represents an in vitro fluidic model of the small intestine barrier, where a 3D reconstructed intestinal epithelium is integrated with MIVO® which simulates the physiological stimuli of the intestinal environment.
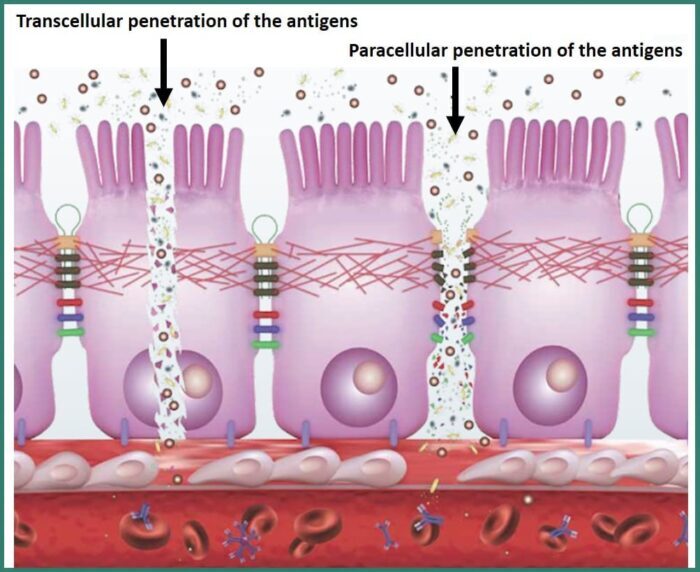
Through this system, the absorption of two non-metabolized sugars, lactulose and mannitol, involved in two different routes of drug absorption, transcellular and paracellular, was studied: this approach allows to monitor these two different absorption mechanisms over time, and their ratio, which is commonly analyzed in clinic procedures to evaluate the patient’s intestinal permeability and therefore his health condition.
The intestinal absorption study was carried out by simulating conditions of healthy and pathological tissue: in the latter case the barrier properties of the intestinal epithelium were compromised with the aim of evaluating the ability of the damaged tissue to undergo a process of self- healing in a fluid-dynamic environment.
The results showed that an in vivo-like plateau of the percentage of absorbed sugars was reached for lactulose and mannitol and that the mannitol absorbed was much greater than the lactulose, in line with clinical data, thus revealing the good reliability of the model. Interestingly, after the damage, the permeability of the intestine and the relative pattern of lactulose passage was completely restored only in dynamic conditions within MIVO® chamber, due to the beneficial role of the dynamic fluid flow beneath the membrane, as explained below.
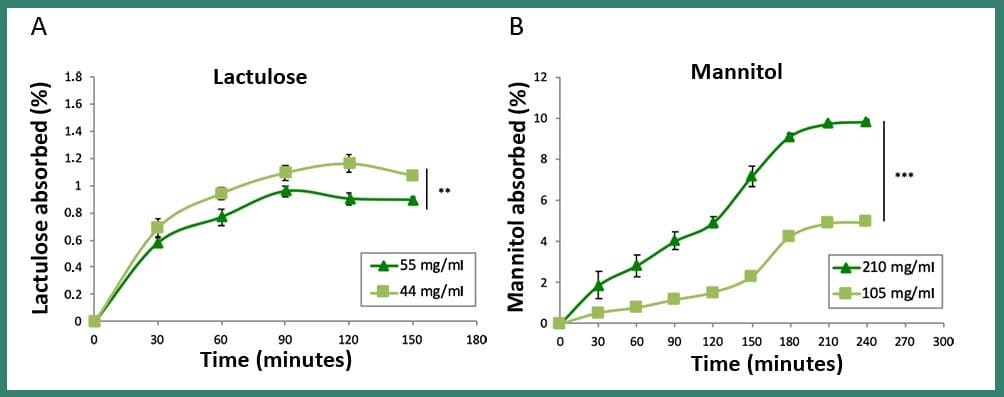
Fig3: Comparison between the absorption of two different concentrations of lactulose (A) and mannitol (B) in dynamic conditions (MIVO® device). These results show that the absorption path of the two sugars is profoundly different, as confirmed by the clinical procedure, where these absorption data taken from urine tests are used to study the possible intestinal damage of patients
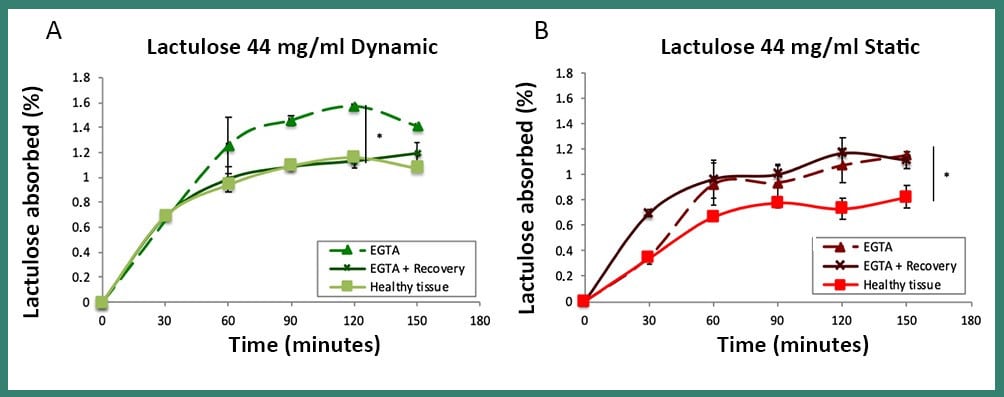
Fig4: Absorption of lactulose in dynamic (A) and static (B) conditions, in the healty, damaged (EGTA) and recovered (EGTA + Recovery) tissue. The passage of lactulose, which had previously been observed in healthy tissue, was completely restored exclusively under dynamic conditions within the MIVO® chamber, proving that the static model does not provide a good simulation of the tissue microenvironment
The intestinal epithelium is a highly specialized tissue, formed by different cell populations in continuous exchange, and, for this reason, it has the ability to quickly restore its integrity following an injury. This study showed that, in damaged intestinal tissue, , the pattern of lactulose passage, displayed in healthy one, was completely restored only in dynamic conditions within MIVO® chamber.
This is probably due to the beneficial role of the dynamic flow of the cell culture medium beneath the tissue, capable of removing waste accumulation and improving both system mass transport and cell turnover, thus contributing to its healing. In fact, the accumulation of waste products, mainly lactate, which can occur in two-dimensional static cultures, can lead to an acidification of the pH in the tissue microenvironment, thus compromising cell growth.
The alteration of permeability is the basis of the pathogenesis of many gastroenterological and non-gastro pathologies, including not only infectious enterocolitis and chronic intestinal inflammatory diseases (IBD), which include ulcerative colitis (UC) and Crohn’s disease (CD), but also irritable bowel syndrome (IBS), bacterial overgrowth syndrome of the small intestine (SIBO), celiac disease, food intolerances and even atopic manifestations. In this context, therefore, the in vitro modeling of a fluid-dynamic human intestine environment offers an innovative and useful approach for study the recovery of the physiological function of the intestinal barrier following damage/injury.
In conclusion, the results, here summarized, indicate that the MIVO® combined with a 3D human intestinal tissue can be a reliable in vitro model of healthy and pathological small intestinal barrier to study the passage of molecules, by discriminating the two main intestinal absorption mechanisms. Moreover, this system could become a useful tool to test the effects of therapies and drugs on the correct functioning of the intestinal barrier.
Ilaria Pulsoni