Cavo M, Fato M, Peñuela L, Beltrame F, Raiteri R, Scaglione S, describes a 3D cell laden breast cancer model
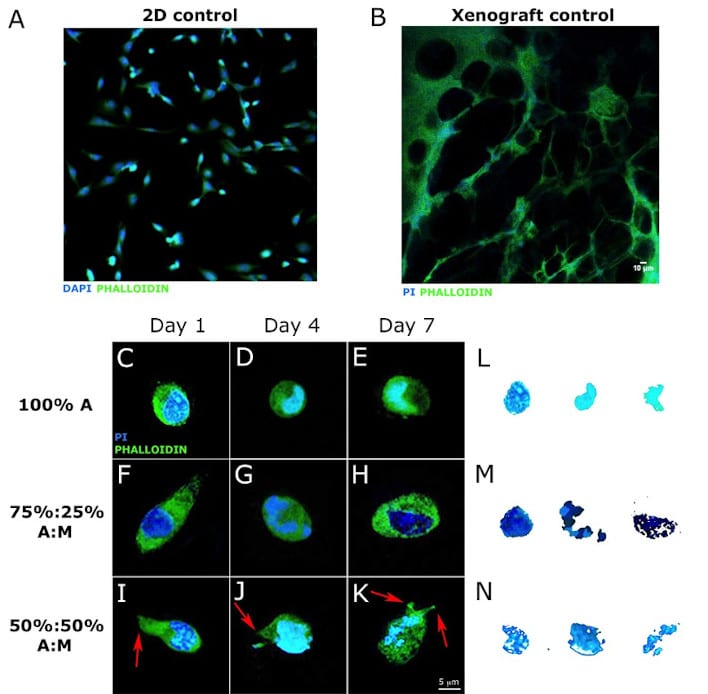
Mechanical stimuli from the cell microenvironment play a key role in affecting several types of cell behaviour, both in healthy and in pathological conditions. In particular, cells sense their microenvironment via trans-membrane proteins and consequently regulate several physiological processes such as migration, proliferation, differentiation, morphology and gene expression, as well as the response to drugs. In vivo, cells are embedded within a complex three-dimensional gel – the Extracellular Matrix (ECM) – that provides mechanical support while directing cellular behavior. Interestingly, more and more literature show that the ECM also plays a relevant role in the onset of a considerable number of diseases: for example, it has been shown that ECM biomechanical properties directly influence and are influenced by the progression of neoplastic disease while, during metastatic invasion, ECM rigidity can affect the motility of carcinoma cells. In a research study published in Scientific Reports authors developed mechanically-tuned alginate hydrogels to study the role of substrate elasticity on breast adenocarcinoma cell activity. The hydrogel elastic modulus was measured via atomic force microscopy and a remarkable range (150-4000 kPa) was obtained. A breast cancer cell line, MCF-7, was seeded within the 3D gels, on standard Petri and alginate-coated dishes (2D controls). Cells showed dramatic morphological differences when cultured in 3D versus 2D, exhibiting a flat shape in both 2D conditions, while maintaining a circular, spheroid-organized (cluster) conformation within the gels, similar to those in vivo. Moreover, we observed a strict correlation between cell viability and substrate elasticity; in particular, the number of MCF-7 cells decreased constantly with increasing hydrogel elasticity. Remarkably, the highest cellular proliferation rate, associated with the formation of cell clusters, occurred at two weeks only in the softest hydrogels (E = 150-200 kPa), highlighting the need to adopt more realistic and a priori defined models for in vitro cancer studies.
M. Cavo, M. Fato, L. Peñuela, F. Beltrame, R. Raiteri, S. Scaglione (2006): “Microenvironment complexity and matrix stiffness regulate breast cancer cell activity in a 3D in vitro model”. Sci Rep.
All Resources
Never stop learning!
Check publications from the team, protocols, and useful information to boost your research and get into organ on chip technology!