The team describes a Clinical-Relevant reliable 3D neuroblastoma Model towards immunotherapies testing
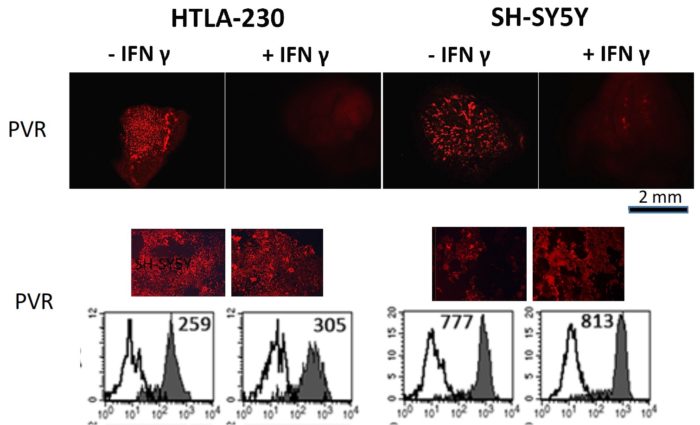
Neuroblastoma (NB) represents an aggressive, metastatic solid tumors of childhood, with a very poor survival rate, despite the use of combination therapies including novel immunotherapies. Treatment failures are mainly due to the lack of adequate in vitro models for studying the efficacy of potential therapeutics, including those aimed to enhance anti-tumor immune responses. A recent study published in Frontiers in Immunology shows a 3D cell laden hydrogel as tumor model to evaluate the effects of the three-dimensionality on biological and immunological properties of NB cells. This 3D model highlighted molecular features that more closely resemble the immunophenotypic variants occurring in vivo and not fully appreciated in classical 2D culture conditions. In particular, NB cell lines grown within the 3D alginate spheres presented spheroid morphology, optimal survival, and proliferation capabilities. 3D cultured NB cells were also evaluated for the constitutive and IFN-γ-induced expression of surface molecules capable of tuning the anti-tumor activity of natural killer (NK) cells including immune checkpoint ligands. In particular, IFN-γ induced de novo expression of high amounts of HLA-I molecules, increased the expression of the immune checkpoint ligands PD-Ls and B7-H3 while virtually abrogating that of PVR, whose expression correlates with high susceptibility to NK-mediated killing. Thus, this 3D cell laden hydrogel might represent a clinical-relevant cell culture platform where to test the efficacy of personalized therapeutic approaches aimed to optimize the current and innovative immune based therapies in a very systematic and reliable way.
A. Marrella, A. Dondero, M. Aiello, B. Casu, D. Olive, S. Regis, C. Bottino, D. Pende, R. Meazza, G. Caluori, R. Castriconi and S. Scaglione (2019): “Cell-Laden Hydrogel as a Clinical-Relevant 3D Model for Analyzing Neuroblastoma Growth, Immunophenotype, and Susceptibility to Therapies”. Front. Immunol.
All Resources
Never stop learning!
Check publications from the team, protocols, and useful information to boost your research and get into organ on chip technology!