The development of effective cancer therapeutics faces significant challenges during the preclinical phase, where traditional in vivo models, while valuable, are often time-consuming, costly, and ethically complex. Moreover, they frequently fail to capture the nuances of human physiology, leading to discrepancies between preclinical results and clinical outcomes.
In recent years, the scientific community has increasingly focused on developing advanced in vitro systems that can bridge this gap, aiming to not only reduce animal use but also provide more predictive and physiologically relevant data.
Our recent study, published in ALTEX and entitled “3D fluid-dynamic ovarian cancer model resembling systemic drug administration for efficacy assay,” presents a new step forward in this direction. We developed a 3D ovarian cancer model integrated into the MIVO® (Multi In Vitro Organ) fluidic device, creating a dynamic system that mimics human blood circulation and drug perfusion through tumor tissue.
At the core of our work is a hydrogel-based 3D tumor construct designed to reproduce the architecture and microenvironment of ovarian cancer tissue. The key innovation lies in the dynamic conditions provided by the MIVO® device, which significantly improved the model’s ability to predict in vivo drug responses.
This enables the simulation of systemic drug administration, allowing compounds to diffuse through the model as they would in the bloodstream.
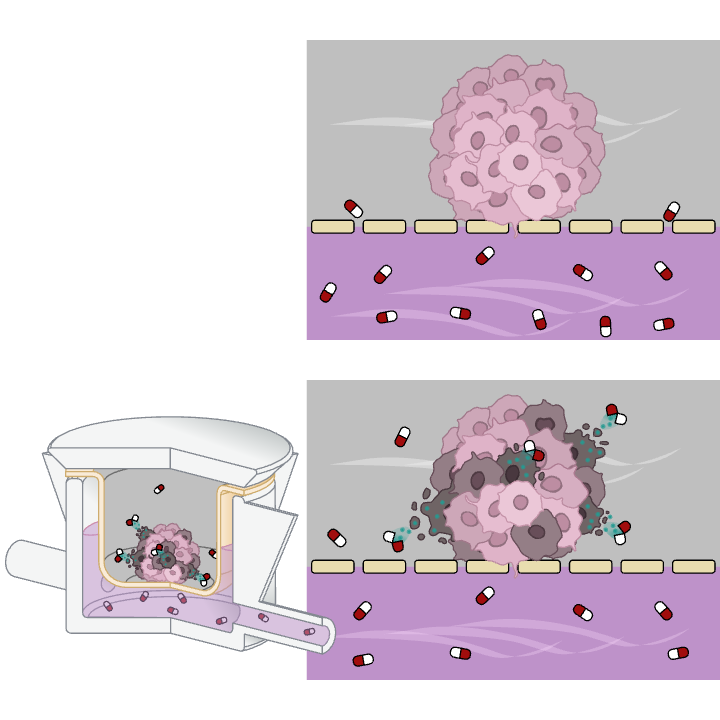
Dynamic Conditions Drive Predictive Accuracy
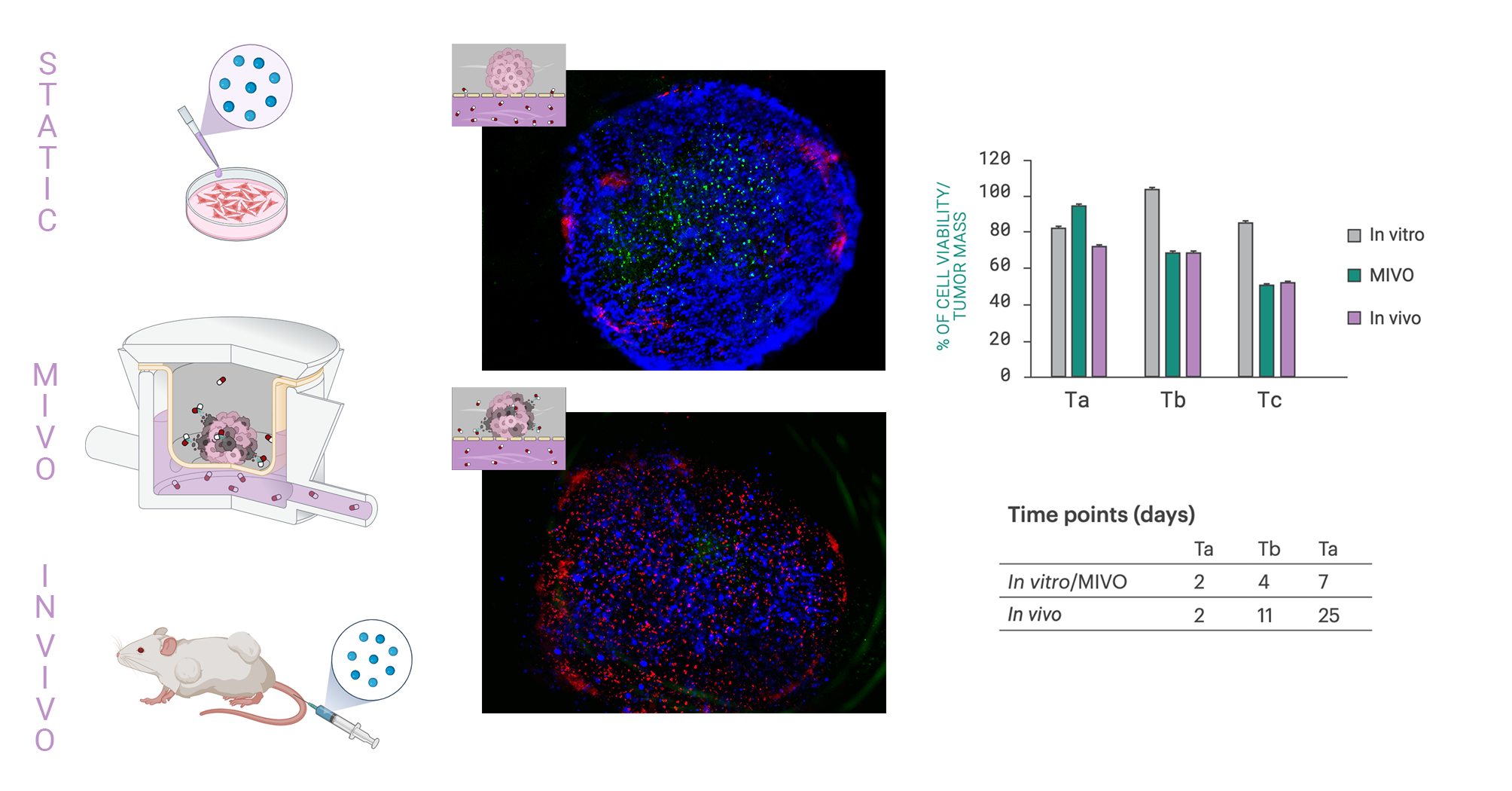
The introduction of fluid dynamics fundamentally changed the model’s predictive capacity. Comparative analyses between static in vitro setups, MIVO® dynamic cultures, and in vivo xenograft models revealed indeed a crucial distinction: only the dynamic MIVO® system reproduced the tumor response observed in animals. Static models failed to capture the complex interplay of diffusion, transport, and cellular response that defines real tumor physiology.
Under dynamic perfusion, apoptotic cells were uniformly distributed throughout the 3D model, indicating consistent therapeutic action. In static conditions, however, apoptosis was limited to the outer layers, with the core remaining largely proliferative.
Furthermore, while traditional in vivo studies required about five weeks to observe significant tumor reduction, the MIVO® dynamic model achieved comparable efficacy outcomes in just one week. This acceleration is not merely a matter of speed, but reflects the improved drug transport and penetration made possible by the dynamic environment.
High-performance liquid chromatography (HPLC) analyses revealed that under static conditions, drug concentration gradients formed within the 3D structure, resulting in uneven exposure and inconsistent cell viability results. In contrast, the MIVO® system ensured uniform cisplatin distribution across the entire hydrogel volume.
Toward Faster, More Ethical, and Predictive Drug Testing
The implications of these findings are substantial. By producing in vivo-comparable efficacy data in one-fifth the time of traditional animal studies, this combined 3D–MIVO® system represents a major leap in preclinical testing efficiency. It allows researchers to assess therapeutic efficacy rapidly and accurately, reducing not only the duration and cost of early drug development but also the ethical burden associated with animal experimentation.
While our study focused on ovarian cancer, the principles demonstrated here are broadly applicable. The combination of 3D tissue engineering and dynamic perfusion can be adapted to model various solid tumors or even other disease contexts where perfusion and systemic drug exposure play critical roles.
This research showcases how the integration of biology and microfluidics can transform the way we test and understand cancer therapeutics. By faithfully reproducing human physiological conditions, the MIVO® system stands as a promising bridge between traditional in vitro and in vivo models — a step toward faster, more reliable, and more ethical drug discovery.

