Immuno-oncology is the study of the pathways that lead to the genesis and progression of cancer, with the goal of developing possible therapeutics to prevent or stop cancer from evading the immune system.
In our body there are cells that have the potential to turn cancerous. These typically develop a particular biochemical and biophysical signature that makes them discoverable by the immune system. In fact, immune cells can recognize external threats and distinguish normal to altered cell types. Nevertheless, sometimes malignant cells manage to avoid detection by our immune system and thus become a threat to our health.
In this context, immunotherapies are emerging as promising strategies to cure cancer and extend patients’ survival. These advanced therapies use different strategies to augment or re-establish the immune system’s ability to prevent and fight cancer: the reintroduction of a patient’s own immune cells harvested from tissue extracted from their tumour(s), also known as cell therapy; the creation in the lab of targeted antibodies able to bind to specific targets on cancer cells and thereby trigger an immune response or cancer vaccines, which prevent the onset of cancer by stimulating an immune response against cancer cells.
Significant progress has been made by using these strategies clearly demonstrating the feasibility of manipulating immune cells to selectively target cancerous cells, often achieving better clinical outcomes, and improving quality of life in comparison to conventional therapies.
For all main types of immunotherapies preclinical trials had doubled, JRC reported in the Advanced Non-animal Models in Biomedical Research (Immuno-oncology) Executive Summary (EUR 30334/3 EN). However, major scientific hurdles still need to be faced to achieve significant clinical impacts. One is the development of reliable preclinical models capable of capturing all the biological intricacies of the tumor milieu and the immune system, overcoming the limitations of traditional systems.
Indeed, although mouse models are widely used in cancer research, they lead to species-specific responses that often misrepresent human biology, resulting in poor translation of research results into the clinic. Likewise, 2D standard monolayers are flat cost-effective systems that cannot replicate the complexity of the in vivo scenario, mainly due to the bi-dimensionality resulting in the lack of proper cell-to-cell and cell-to-ExtraCellular Matrix reciprocal interactions. To solve these constraints, several 3D in vitro culture models have been realized to carry out advanced preclinical experimental investigations under more physiological conditions.
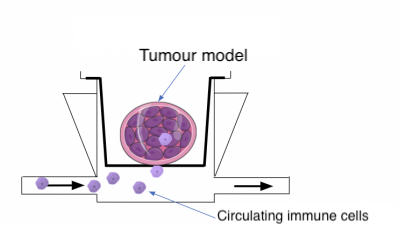
Our patented MIVO technology is a fluidic technology allowing to recreate both the three-dimensional complexity of the tumour and the microcirculation that feeds the tissue, reproducing the dinamicity of the in vivo condition. On the one hand, our multi-chamber fluidic platform is suitable for hosting 3D matrix-assisted tissues (e.g hydrogels), as well as for the employment of tumour biopsies of clinically relevant size.
Moreover, by connecting the MIVO with a pumping system, it is possible to mimic the systemic circulation, in a highly controlled manner, allowing to study in vitro the survival, escape, and activation of immune cells during their journey in the vascular network.
Check our latest review article to get an overview on the main challenges and possible future trends in the immune-oncology scenario.
In this work, we focused on 3D models based on different types of polymers, and we introduced the possibility to combine them with technological fluid-dynamic platforms like MIVO, reproducing the complex interactions between tumor cells and immune effectors migrated in situ via the systemic circulation, pointing out the challenges that still have to be overcome for setting more predictive preclinical assays.
Tumor Microenvironment and Hydrogel-Based 3D Cancer Models for In Vitro Testing Immunotherapies

