The small intestine is a central organ in human physiology: in addition to its pivotal role in nutrient uptake, it is the principal site of drug absorption. Yet, despite tremendous advances in biomedical research, traditional static cell culture systems fail to capture the dynamic nature of the gut, which experiences constant fluid flow, mechanical forces, and cell-cell interactions. In this context, the recent work by researchers in React4life introduces an elegant and powerful solution: a dynamic double-flow gut-on-chip platform built upon the MIVO® millifluidic device, which more closely mimics the physiological milieu of the human gut. The development of the MIVO® Double Flow system represents a significant leap forward in this field, offering a more physiologically relevant model for studying gut barrier function, drug absorption, and disease mechanisms.
Gut-on-chip innovation: fast maturation and in vivo-like permeability
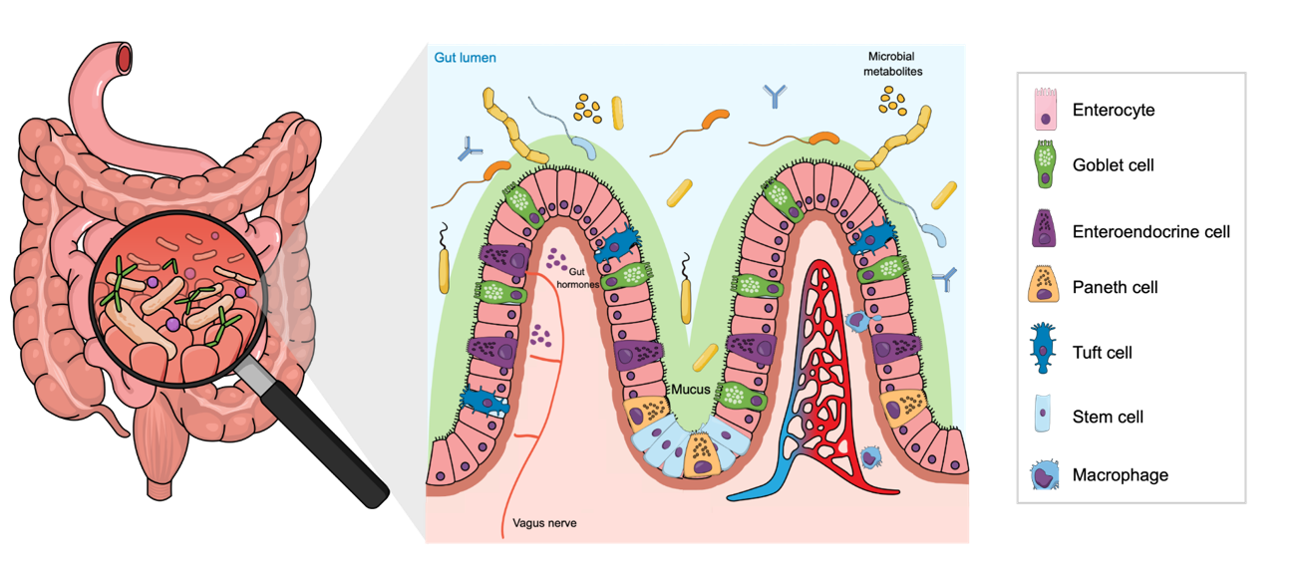
At the heart of this study is a clever design: a co-culture of two human intestinal cell lines, CaCo-2 and HT-29, under continuous flow conditions. These cell lines, when grown together, can recapitulate important features of the epithelium, CaCo-2 contributing absorptive enterocyte-like cells, and HT-29 contributing goblet-like mucus-secreting cells. By adjusting the ratio of CaCo-2 to HT-29 cells, the authors were able to finely tune the thickness of the mucus layer and the barrier properties of the epithelium.
A particularly striking result is how the MIVO®-enabled dynamic culture accelerates cell maturation: the study report that in MIVO® double-flow gut-on-chip, a fully differentiated intestinal layer (as assessed by metrics such as transepithelial electrical resistance (TEER), expression of tight junction proteins like Zonula Occludens-1, and Alcian blue staining for mucus) is achieved in just 7–10 days, compared to about 21 days in traditional static culture.
But the innovation does not stop at basal flow. The model incorporates a second flow circuit on the apical side, simulating the luminal (intestinal) side, while another flow runs underneath (basolateral), resembling the bloodstream. This “double-flow” architecture means that both the lumen and the “blood” compartments are under continuous perfusion, which is more physiologically realistic.
Importantly, this design yields intestinal permeability characteristics that more closely resemble in vivo behaviour. In other words, the model does not just look like a gut barrier: it behaves like one, in terms of absorption dynamics.

Why MIVO® Gut-on-chip stands out among the currently available models
With the growing demand for physiologically relevant gut-on-chip technologies, the MIVO® platform offers a dynamic and highly versatile intestinal model that overcomes the limitations of static Transwells and traditional in vitro systems. Its unique double-flow configuration, complete with continuous apical perfusion, enables realistic luminal conditions, improved compound handling and advanced applications ranging from drug absorption prediction to microbiota research.
Below are the key advantages that make the MIVO® Gut-on-Chip one of the most powerful and application-ready intestinal models currently available:
- Double-flow gut-on-chip to mimic both luminal and capillary dynamics: Unlike conventional systems, MIVO® features continuous apical flow, a major advantage for simulating luminal conditions, improving shear stress, and maintaining epithelial polarization. The apical perfusion allows controlled delivery of nutrients, drugs and biological inputs, making the model far closer to true human intestinal physiology.
- Fast intestinal barrier maturation for high-throughput gut-on-chip studies: while conventional static systems typically require around 21 days to form a functional intestinal barrier, the MIVO® double-flow system achieves comparable or superior maturation within 7–10 days. This rapid barrier formation is essential for researchers seeking high-throughput gut-on-chip assays, early-stage drug screening, and time-sensitive permeability studies, reducing both operational time and experimental costs.
- Ideal for poorly soluble compounds and complex formulations: the presence of an apical flow enables the handling of low-solubility molecules that normally precipitate or stratify in static wells, such as cyclosporine, probucol, fenofibrate, curcumin, resveratrol, or lipid-based formulations. Dynamic flow improves compound dispersion, maintaining stable luminal concentrations and enabling reproducible absorption measurements.
- Microbiota-ready platform for host–microbe interaction studies: the controlled apical compartment allows the introduction of commensal or engineered bacterial strains, enabling studies on microbiota–epithelium interplay, mucosal immune responses, probiotic testing, or the impact of microbial metabolites on epithelial permeability and drug transport.
- Tunable mucus-secreting barrier for disease and absorption models: by adjusting the CaCo-2/HT-29 ratio, the MIVO® platform allows modulation of mucus thickness and tight-junction integrity, making it suitable for modeling leaky gut conditions, high-mucus barriers, inflammatory scenarios, variable absorption phenotypes relevant to oral delivery.
- Predictive drug absorption aligned with in vivo data: the double-flow design produces permeability profiles that correlate more tightly with human physiology than static models. This improves the predictivity of drug absorption, toxicology, nutraceutical uptake, and bioavailability assessment.
- Reduced animal use through human-relevant intestinal physiology: MIVO® replaces low-relevance animal tests with a more accurate human barrier model, reducing ethical burden and increasing translational reliability.
- Modular and scalable for multi-organ and integrated ADME studies: its compact and flexible architecture makes MIVO® suitable for multi-organ-on-chip setups, absorption–metabolism workflows, and combined GI–liver pipelines. This scalability supports pharmaceutical applications from early discovery to mechanistic studies.
In summary, this work demonstrates a compelling combination of engineering elegance and biological fidelity. By integrating double-flow dynamics, tunable mucus production, and physiologically relevant barrier function, the MIVO® Gut-on-Chip establishes a new benchmark for intestinal in vitro models. Its predictive absorption performance, compatibility with complex formulations and microbiota, and rapid maturation kinetics open the door to faster, more human-relevant research pipelines. As such, this platform not only advances drug absorption and disease modeling studies, but also contributes meaningfully to reducing reliance on animal experimentation and accelerating the transition toward next-generation, human-centric testing strategies.
References:
MEF Palamà, M Aiello, G Borka, J Furci, I Parodi, G Firpo, S Scaglione. A Dynamic Double‐Flow Gut‐On‐Chip Model for Predictive Absorption Studies In Vitro. Adv. Mater. Technol. 9/2025

