Cancer research has undergone a transformative shift in recent decades, moving beyond traditional approaches to embrace the intricate dance between tumors and the immune system. As our understanding of cancer biology deepens, it has become increasingly clear that the immune system plays a pivotal role in both tumor progression and regression. This realization has propelled cancer immunology to the forefront of oncological research, with scientists working tirelessly to unravel the complex interactions between immune cells and cancer cells. However, traditional research models often fall short in capturing the dynamic, three-dimensional nature of these interactions within the tumor microenvironment. As the field pushes toward precision cancer immuno-oncology, next-generation human-relevant preclinical models are urgently needed.
MIVO® immune-on-chip: a physiologically relevant platform for immune–tumor crosstalk
The MIVO® platform represents a significant leap forward in creating physiologically relevant models for studying immune cell behavior within tumor environments. Unlike conventional two-dimensional cell cultures or animal models, MIVO® provides a humanized, fluid-dynamic, and three-dimensional environment that accurately mimics the conditions found within the human body.
This technological advancement allows observing immune cell migration, infiltration, and cytotoxic activity in a context that closely resembles in vivo conditions
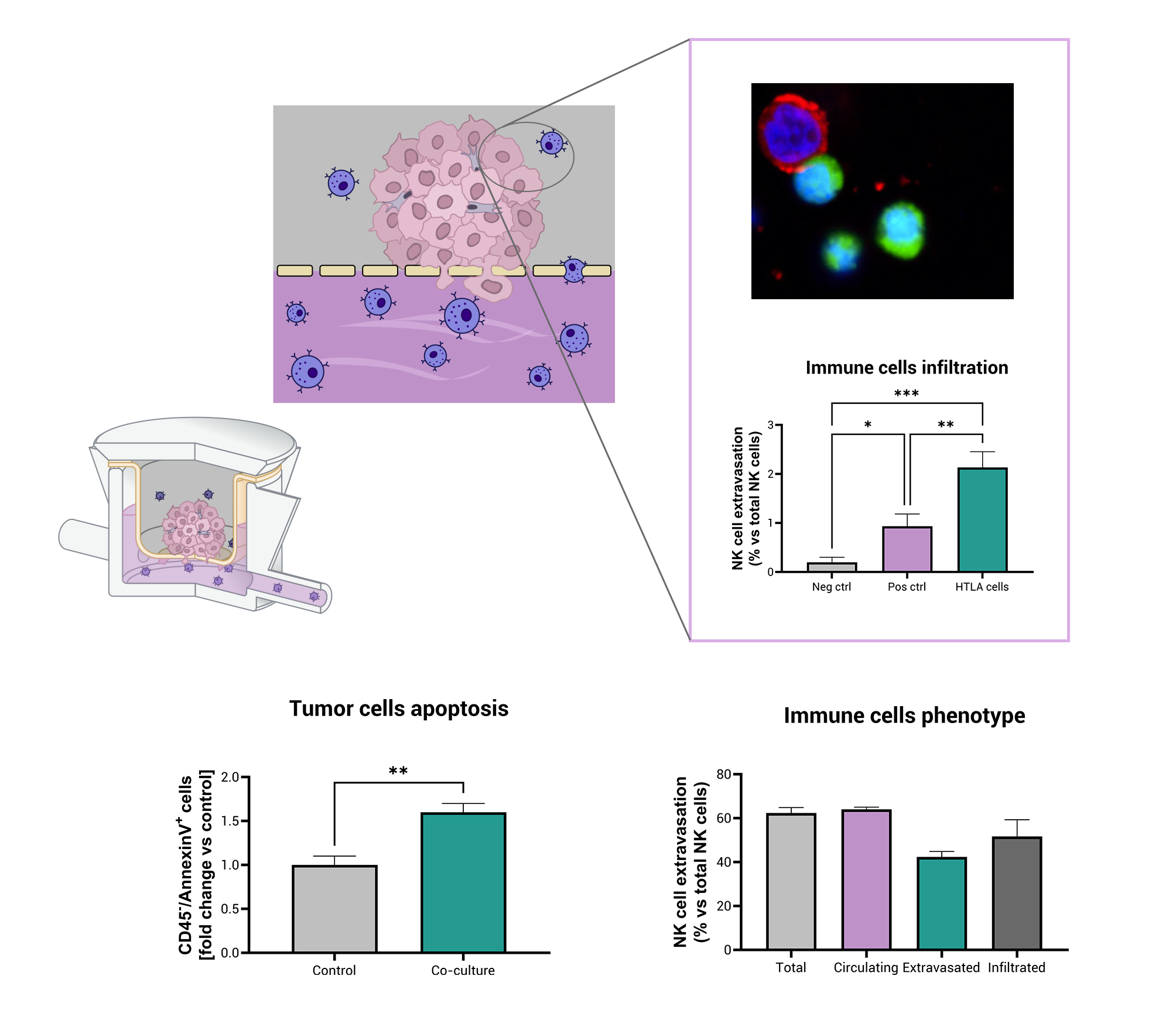
At its core, the MIVO® platform is engineered to mimic circulating immune cells interacting with three-dimensional tumor tissues, reproducing blood-flow-like conditions and realistic infiltration events. In a recently published study in collaboration with University of Genoa and IRCCS Istituto Giannina Gaslini (Genoa), we used aggressive neuroblastoma (HTLA-230) tumor cells embedded in a 3D alginate scaffold positioned above a dynamic circuit carrying human natural killer (NK) cells. Over just four hours, the system revealed a tumor-specific extravasation of NK cells and their infiltration into the tumor construct. Importantly, the platform demonstrated that NK cells were capable of entering the alginate matrix, migrating toward tumor cells and establishing contact with them, a process rarely observed in standard 2D culture systems. Confocal microscopy confirmed that CFSE-labelled NK cells (green) were found among GD2-positive tumor cells (red) within the scaffold.
Moreover, the model showed a significant induction of tumor-cell apoptosis: tumor cells recovered from the dynamic co-culture displayed markedly higher Annexin-V positivity, indicating that the infiltrated NK cells retained cytotoxic function within the 3D environment.
In parallel, the NK cell phenotype was investigated, and while roughly 70% of NK cells in circulation expressed CD16 (a marker associated with high cytotoxic potential), the fraction of CD16-positive NK cells in the extravasated and infiltrated populations was strongly reduced. This observed shift toward a CD16-negative phenotype among infiltrating NK cells is consistent with findings in various solid-tumor settings, where infiltrated NK cells frequently show reduced CD16 expression and correlate with poorer clinical outcome.
Crucially, the frequencies of NK cell infiltration in the MIVO® system (only around 2 % of circulating NK cells extravasated toward the tumor compartment) align with the low NK infiltration reported in human tumor tissues, thereby reinforcing the model’s clinical relevance.
Capturing the Complexity of NK-Cell-Driven Anti-Tumor Responses
Unlike static assays, MIVO® enables the study of dynamic and physiologically relevant immune responses within a controlled fluid-dynamic environment, reproducing physiologically-relevant conditions.
- NK cell migration through a matrix resembling human tumor tissue: within this setting, NK cells are not simply placed in contact with tumor cells, but they actively extravasate from a circulating compartment and infiltrate into the tumor matrix. This sequence of events mirrors the critical steps of immune surveillance and tumor targeting observed in vivo, providing a more faithful representation of innate immune behavior against solid tumors.
- Tumor-directed NK extravasation driven by flow-mediated signaling: NK cells respond to fluid-dynamic forces and biochemical gradients to leave the leave the circulating, migrating against the gravity force, and enter the tumor compartment. By recapitulating this directional trafficking process, the system captures recruitment mechanisms usually lost in standard static cultures, thereby enhancing the physiological relevance of immune–tumor studies.
- Cell–cell communication through soluble mediators and cytokines: beyond physical contact, NK cells and tumor cells communicate through a network of secreted factors within the dynamic microenvironment. This continuous exchange of soluble signals supports activation, suppression, and feedback loops, more accurately mirroring the complex regulatory interactions that define real tumor ecosystems.
- Emergence of tumor resistance against NK-mediated cytotoxicity: while infiltrating NK cells can drive tumor apoptosis, the selective pressure they impose may allow more resistant tumor subsets to emerge. Observing this interplay between immune attack and tumor adaptation is crucial, as it reflects clinically relevant immune-escape dynamics and supports the identification of combination strategies to maintain durable immune responses.
- Modulation of NK invasion by tumor-derived immunosuppressive molecules: tumor-secreted factors known to orchestrate immune evasion, such as TGF-β, MIF, and VEGF, actively influence NK infiltration and function within the system. This demonstrates that the platform does not merely support immune cell entry but also faithfully reproduces the inhibitory pressures of the tumor microenvironment, enabling the investigation of immunosuppressive pathways and potential interventions to counteract them.
Looking Ahead: Toward Personalized Immune-Oncology Modeling and Immunotherapy Testing
Future iterations of the MIVO® platform are expected to integrate additional components of the tumor microenvironment, including stromal elements, vascular cells, and immunosuppressive mediators, further enhancing biological fidelity. This evolution will strengthen the system’s capacity to reproduce patient-specific immune responses, representing an important step toward truly personalized cancer-modeling strategies.
As the immune-oncology landscape progresses, technologies like MIVO® provide a unique window into real-time cancer-immune dynamics under human-relevant conditions. By enabling controlled and reproducible investigation of immune infiltration, tumor escape, and phenotypic plasticity, the platform accelerates mechanistic discovery and supports the rational design of next-generation therapeutic approaches.
Crucially, this organ-on-chip system is not only a research tool, but also represents a transformative preclinical testing platform. By allowing the functional evaluation of immunotherapies, including cell-based products and biologics, directly within a dynamic human-mimicking tumor environment, MIVO® bridges the gap between mechanistic research and therapeutic validation. This capability opens the door to more accurate prediction of treatment responses, reduced reliance on animal models, and more efficient optimization of immunotherapeutic strategies.

